Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis
- PMID: 40869059
- PMCID: PMC12386832
- DOI: 10.3390/ijms26167738
Unveiling the Adsorptive Potential of Natural Biopolymers for Olive Mill Wastewater Treatment: A Synergistic Approach Using RSM-BBD, Mixture Design, Kinetics, and Mechanistic Analysis
Abstract
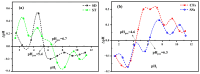
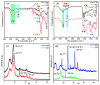
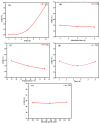
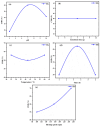
This study evaluates the structural properties and adsorption capacities of four bio-based adsorbents, sawdust (SD), straw (ST), chicken feathers (CFs), and shrimp shells (SSs), for chemical oxygen demand (COD) removal from olive mill wastewater (OMW). Response Surface Methodology (RSM) with a Box-Behnken Design (BBD) was applied to optimize the operational parameters, resulting in maximum COD uptake capacities of 450 mg/g (SD), 575 mg/g (ST), 700 mg/g (CFs), and 750 mg/g (SSs). Among these materials, SSs exhibited the highest COD removal efficiency of 85% under optimal conditions (pH 8, 20 g/L, 30 °C, 5 h, 111 rpm). A mixture design approach was then used to explore the synergistic effects of combining lignocellulosic (SD and ST), chitin-based (SSs), and keratin-based (CFs) adsorbents. The optimized blend (SD 10%, ST 28.9%, SS 38.3%, and CF 22.6%) achieved a COD removal efficiency of 82%, demonstrating the advantage of using mixed biopolymer systems over individual adsorbents. Adsorption mechanisms were investigated through isotherm models (Langmuir, Freundlich, Temkin, and Redlich-Peterson) and kinetic models (pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion). Lignocellulosic adsorbents predominantly followed physisorption mechanisms, while chitin- and keratin-rich materials exhibited a combination of physisorption and chemisorption. Thermodynamic analysis confirmed the spontaneous nature of the adsorption process, with SSs showing the most favorable Gibbs free energy (ΔG = -21.29 kJ/mol). A proposed mechanism for the adsorption of organic compounds onto the bio-adsorbents involves hydrogen bonding, electrostatic interactions, π-π interactions, n-π stacking interactions, hydrophobic interactions, and van der Waals forces. These findings highlight the potential of biopolymer-based adsorbents and their optimized combinations as cost-effective and sustainable solutions for OMW treatment.
Keywords: COD; adsorption; chitin; keratin; lignocellulose; olive mill wastewater (OMW).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Liu T., Ren X., Soundari P.G., Chen H., Awasthi S.K., Varjani S., Pandey A., Zhang Z., Awasthi M.K. Biomass, Biofuels, Biochemicals. Elsevier; Amsterdam, The Netherlands: 2021. Waste Biorefinery Development Toward Circular Bioeconomy with a Focus on Life-Cycle Assessment; pp. 199–230.
-
- Rout P.R., Goel M., Pandey D.S., Briggs C., Sundramurthy V.P., Halder N., Mohanty A., Mukherjee S., Varjani S. Technological Advancements in Valorisation of Industrial Effluents Employing Hydrothermal Liquefaction of Biomass: Strategic Innovations, Barriers and Perspectives. Environ. Pollut. 2023;316:120667. doi: 10.1016/j.envpol.2022.120667. - DOI - PubMed
-
- Moussaid D., Moumnani F.T., Tanji K., Tayibi S., Benabdallah A.C., Kherbeche A., Barakat A., Beniazza R. Efficient Polyphenols Compounds Photodegradation from Olive Mill Wastewater Using Solar-Light-Driven β-Cu2V2O7 and Cu3V2O8 Nanoparticles. J. Water Process Eng. 2025;71:107326. doi: 10.1016/j.jwpe.2025.107326. - DOI
-
- Fernandes M.J., Gomes J., Carvalho P., Martins R.C., Domingues E. Removal and Recovery of Phenolic Compounds from OMW by a Cationic Resin. Chem. Eng. Sci. 2024;291:119925. doi: 10.1016/j.ces.2024.119925. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

