Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst
- PMID: 40869070
- PMCID: PMC12386328
- DOI: 10.3390/ijms26167751
Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst
Abstract
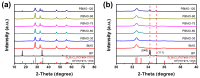
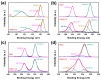
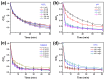
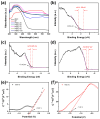
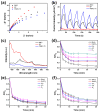
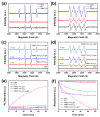
Fenton oxidation technology utilizing hydrogen peroxide is recognized as an effective method for producing reactive oxygen species (ROS) to facilitate the degradation of antibiotics. However, the requirement for strongly acidic conditions during this process significantly restricts its broader applicability. In this study, we synthesized black phosphorus (BP) nanosheets by exposing the {010} crystal planes and then constructed a 0D/2D BP/Bi2MoO6 (PBMO) heterojunction to function as a Fenton catalyst. The PBMO-75 heterojunction exhibited a remarkable increase in photo-Fenton catalytic activity towards oxytetracycline (OTC) under neutral conditions, achieving catalytic efficiencies that were 20 and 8 times greater than those of BP and Bi2MoO6 (BMO), respectively. This can be attributed to its strong absorption of visible light, the establishment of an internal electric field (IEF) at the interface, and the implementation of a Z-scheme catalytic mechanism. Additionally, the photo-Fenton system was further improved in OTC degradation through the continuous conversion of Mo6+/Mo5+ under visible light irradiation in conjunction with H2O2. Based on ERS, XPS, and active species trapping experiments, we propose a Z-scheme charge transfer mechanism for PBMO. This research offers compelling evidence that 0D/2D Z-scheme heterojunctions are promising candidates for the photo-Fenton treatment of antibiotic contaminants.
Keywords: BP/Bi2MoO6; Z-scheme heterojunction; oxytetracycline; photo-Fenton degradation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Construction of novel interface tightly coupled S-scheme heterojunction: Photo-Fenton catalysis for efficient degradation of antibiotics and DFT calculation.Environ Res. 2025 Oct 1;282:122093. doi: 10.1016/j.envres.2025.122093. Epub 2025 Jun 6. Environ Res. 2025. PMID: 40482620
-
Z-scheme heterojunction composed of Fe-doped g-C3N4 and Bi2MoO6 for photo-fenton degradation of antibiotics over a wide pH range: Activity and toxicity assessment.Environ Res. 2024 Jul 1;252(Pt 2):118886. doi: 10.1016/j.envres.2024.118886. Epub 2024 Apr 6. Environ Res. 2024. PMID: 38583659
-
Enhanced built-in electric fields in novel Bi2MoO6/graphdiyne S-scheme heterojunction enable superior molecular oxygen activation for broad-spectrum antibiotic degradation.J Colloid Interface Sci. 2025 Dec 15;700(Pt 3):138620. doi: 10.1016/j.jcis.2025.138620. Epub 2025 Aug 5. J Colloid Interface Sci. 2025. PMID: 40773881
-
A systematic review on photo-Fenton process as an efficient advanced oxidation for degradation of amoxicillin in aqueous environments.Rev Environ Health. 2022 Apr 18;38(2):313-326. doi: 10.1515/reveh-2021-0155. Print 2023 Jun 27. Rev Environ Health. 2022. PMID: 35436046
-
Bismuth-based heterojunction photocatalysts for antibiotic remediation: A review of tetracycline degradation and mechanistic insights.J Environ Manage. 2025 Aug 29;393:127010. doi: 10.1016/j.jenvman.2025.127010. Online ahead of print. J Environ Manage. 2025. PMID: 40884951 Review.
References
-
- Lu Y., Zhou X., Zheng Y., Yang H., Cao W. How far do we still need to go with antibiotics in aquatic environments? Antibiotic occurrence, chemical-free or chemical-limited strategies, key challenges, and future perspectives. Water Res. 2025;275:123179. doi: 10.1016/j.watres.2025.123179. - DOI - PubMed
-
- Pham M.N., Nishimura F., Lan J.C.W., Khoo K.S. Recent advancement of eliminating antibiotic resistance bacteria and antibiotic resistance genes in livestock waste: A review. Environ. Technol. Innov. 2024;36:103751. doi: 10.1016/j.eti.2024.103751. - DOI
-
- Obi C.C., Abonyi M.N., Ohale P.E., Onu C.E., Nwabanne J.T., Igwegbe C.A., Kamuche T.T., Ozofor I.H. Adsorption of antibiotics from aqueous media using nanocomposites: Insight into the current status and future perspectives. Chem. Eng. J. 2024;497:154767. doi: 10.1016/j.cej.2024.154767. - DOI
-
- Ji J., Li H., Liu S. Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Appl. Sci. 2025;15:5182. doi: 10.3390/app15095182. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

