Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages
- PMID: 40869102
- PMCID: PMC12386841
- DOI: 10.3390/ijms26167781
Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages
Abstract
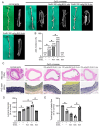
Abdominal aortic aneurysm (AAA) is a life-threatening vascular disease characterized by chronic inflammation, extracellular matrix degradation, and smooth muscle cell apoptosis. Porphyromonas gingivalis (P. gingivalis), a key periodontal pathogen, has been implicated in the progression of cardiovascular diseases, including AAA, but the underlying mechanisms remain unclear. In this study, we investigated the role of GroEL, a bacterial heat shock protein 60 homolog derived from P. gingivalis, in AAA development. We employed a CaCl2-induced AAA mouse model to evaluate the in vivo effects of GroEL. Mice received periaortic CaCl2 application followed by intravenous injections of recombinant GroEL. Histological analyses were performed to assess aneurysmal dilation, elastin degradation, and inflammatory cell infiltration. Flow cytometry and immunohistochemistry were used to determine macrophage phenotypes, while cytokine profiles were quantified via ELISA. In vitro, THP-1 monocytes were treated with GroEL to evaluate its impact on macrophage polarization and cytokine expression. Our results showed that GroEL administration significantly enhanced aortic diameter expansion and elastin breakdown, accompanied by increased infiltration of M1-like macrophages and elevated levels of pro-inflammatory cytokines such as TNF-α and IL-6. In vitro findings confirmed that GroEL promotes M1 polarization and inhibits M2 marker expression in THP-1-derived macrophages. These findings suggest that P. gingivalis-derived GroEL plays a pathogenic role in AAA by modulating macrophage polarization toward a pro-inflammatory phenotype. Targeting microbial components such as GroEL may offer new therapeutic strategies for AAA management.
Keywords: GroEL; IRF5; P. gingivalis; abdominal aortic aneurysm; macrophages; thrombomodulin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Immunoglobulin A/PIGR axis as potential mediators of human abdominal aortic aneurysms revealed by topologically resolved proteomics.J Transl Med. 2025 Jul 7;23(1):747. doi: 10.1186/s12967-025-06758-y. J Transl Med. 2025. PMID: 40624587 Free PMC article.
-
Scoparone alleviates aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching and inflammation via mTOR suppression.J Ethnopharmacol. 2025 Jul 24;351:120080. doi: 10.1016/j.jep.2025.120080. Epub 2025 Jun 7. J Ethnopharmacol. 2025. PMID: 40490231
-
Caspase-11 deficiency ameliorates elastase-induced abdominal aortic aneurysm in mice by suppressing inflammatory response of macrophages.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C93-C106. doi: 10.1152/ajpcell.00716.2024. Epub 2025 Jun 3. Am J Physiol Cell Physiol. 2025. PMID: 40459928
-
Medical treatment for small abdominal aortic aneurysms.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009536. doi: 10.1002/14651858.CD009536.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972146 Free PMC article.
-
Laparoscopic surgery for elective abdominal aortic aneurysm repair.Cochrane Database Syst Rev. 2017 May 4;5(5):CD012302. doi: 10.1002/14651858.CD012302.pub2. Cochrane Database Syst Rev. 2017. PMID: 28471523 Free PMC article.
References
-
- Suzuki J., Aoyama N., Aoki M., Tada Y., Wakayama K., Akazawa H., Shigematsu K., Hoshina K., Izumi Y., Komuro I., et al. Incidence of periodontitis in Japanese patients with cardiovascular diseases: A comparison between abdominal aortic aneurysm and arrhythmia. Heart Vessel. 2015;30:498–502. doi: 10.1007/s00380-014-0507-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

