CO and NO Coordinate Developmental Neuron Migration
- PMID: 40869105
- PMCID: PMC12387027
- DOI: 10.3390/ijms26167783
CO and NO Coordinate Developmental Neuron Migration
Abstract
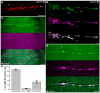
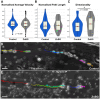
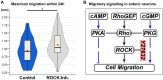
Similarly to the short-lived messenger nitric oxide (NO), the more stable carbon monoxide (CO) molecule can also activate soluble guanylyl cyclase (sGC) to increase cGMP levels. However, CO-induced cGMP production is much less efficient. Using an accessible invertebrate model, we dissect a potential interaction between the canonical NO/sGC/cGMP and CO signalling pathways during development. The embryonic midgut of locusts is innervated by neurons that migrate in four discrete chains on its outer surface. Transcellular diffusing NO stimulates enteric neuron migration via cGMP signalling. The application of an NO donor results in virtually all enteric neurons being cGMP-immunoreactive while CO increases cGMP production only in approximately 33% of the migrating neurons. Cellular CO release appears to act as a slow down signal for motility. We quantify how CO specifically increases the interneuronal distance during chain migration. Moreover, time-lapse microscopy shows that CO reduces the directionality of the migrating neurons. These findings support the function of NO and CO as antagonistic signals for the coordination of collective cell migration during the development of the enteric nervous system. These experiments and the resulting insights into basic scientific questions prove once more that locust embryos are not only preparations for basic research, but also relevant models for screening of drugs targeting NO and CO signalling pathways as well as for isolating compounds affecting neuronal motility in general.
Keywords: chain migration; directionality; enteric nervous system; gaseous messenger signalling; locust embryo; neural development.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Regulation of enteric neuron migration by the gaseous messenger molecules CO and NO.Development. 2009 Jan;136(1):85-93. doi: 10.1242/dev.026716. Epub 2008 Nov 19. Development. 2009. PMID: 19019991
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Expression of soluble guanylate cyclase (sGC) and its ability to form a functional heterodimer are crucial for reviving the NO-sGC signaling in PAH.Free Radic Biol Med. 2024 Nov 20;225:846-855. doi: 10.1016/j.freeradbiomed.2024.11.009. Epub 2024 Nov 6. Free Radic Biol Med. 2024. PMID: 39515593 Free PMC article.
-
Pharmacological approaches to nitric oxide signalling during neural development of locusts and other model insects.Arch Insect Biochem Physiol. 2007 Jan;64(1):43-58. doi: 10.1002/arch.20161. Arch Insect Biochem Physiol. 2007. PMID: 17167749 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

