Cathepsins in Neurological Diseases
- PMID: 40869205
- PMCID: PMC12386458
- DOI: 10.3390/ijms26167886
Cathepsins in Neurological Diseases
Abstract
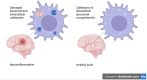
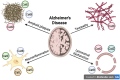
Cathepsins, a family of lysosomal proteases, play critical roles in maintaining cellular homeostasis through protein degradation and modulation of immune responses. In the central nervous system (CNS), their functions extend beyond classical proteolysis, influencing neuroinflammation, synaptic remodeling, and neurodegeneration. Emerging evidence underscores the crucial role of microglial cathepsins in the pathophysiology of several neurological disorders. This review synthesizes current knowledge on the involvement of cathepsins in a spectrum of CNS diseases, including Parkinson's disease, Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis, epilepsy, Huntington's disease, and ischemic stroke. We highlight how specific cathepsins contribute to disease progression by modulating key pathological processes such as α-synuclein and amyloid-β clearance, tau degradation, lysosomal dysfunction, neuroinflammation, and demyelination. Notably, several cathepsins demonstrate both neuroprotective and pathogenic roles depending on disease context and expression levels. Additionally, the balance between cathepsins and their endogenous inhibitors, such as cystatins, emerges as a critical factor in CNS pathology. While cathepsins represent promising biomarkers and therapeutic targets, significant gaps remain in our understanding of their mechanistic roles across diseases. Future studies focusing on their regulation, substrate specificity, and interplay with genetic and epigenetic factors may yield novel strategies for early diagnosis and disease-modifying treatments in neurology.
Keywords: autophagy; cathepsins; neurodegenerative diseases; neuroinflammation; proteolysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical