MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma
- PMID: 40869209
- PMCID: PMC12386254
- DOI: 10.3390/ijms26167889
MicroRNAs and Their Inhibition in Modulating SLC5A8 Expression in the Context of Papillary Thyroid Carcinoma
Abstract
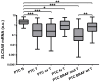
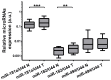
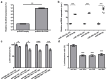
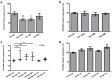
SLC5A8 is a protein coded by the SLC5A8 gene, and has been proposed as a tumor suppressor and iodide transporter. Its expression is reduced in papillary thyroid carcinoma (PTC), yet the mechanisms underlying this phenomenon are largely unknown. We hypothesized that SLC5A8 expression in PTC is reduced by microRNAs and can be modulated by their inhibition. We used real-time PCR to analyze the expression of SLC5A8 and the microRNAs of interest in a set of 49 PTC/normal tissue pairs. We used an in silico approach to identify microRNAs upregulated in PTC and putatively binding to the SLC5A8 transcript. Luciferase assays were performed to confirm the direct binding of synthetic microRNAs to the 3'UTR of SLC5A8. Subsequently, using mir-expressing plasmids and microRNA sponges, including a microRNA sponge designed to simultaneously inhibit three selected microRNAs, we checked the impact of the modulation of microRNAs on endogenous SLC5A8. Finally, we investigated if modulation of SLC5A8 induces changes in transcriptomes. We confirmed the downregulation of SLC5A8 in PTC. In silico analysis revealed microRNAs potentially targeting SLC5A8. Luciferase assay confirmed direct binding between the 3'UTR of SLC5A8 and miR-181a-5p, miR-182-5p, and miR-494-3p. MiR-181a-5p and miR-182-5p were upregulated in PTC. In HEK293 cell lines, transfection with mir-181a- and mir-182-expressing plasmids decreased endogenous SLC5A8 mRNA, while silencing of miR-181a-5p, miR-182-5p, miR-494-3p, and all three microRNAs simultaneously increased SLC5A8 expression; however, only simultaneous inhibition was able to induce changes visible for SLC5A8 protein. Changes in SLC5A8 expression did not alter the whole transcriptome significantly. This study shows microRNA-dependent regulation of SLC5A8 expression and underlines the potential effectiveness of simultaneous inhibition of a few microRNAs to derepress their common target.
Keywords: AIT; PTC; SLC5A8; microRNA; papillary thyroid carcinoma; thyroid carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
FTO-regulated m6A modification of pri-miR-139 represses papillary thyroid carcinoma metastasis.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2025 May 28;50(5):815-826. doi: 10.11817/j.issn.1672-7347.2025.250018. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40916819 Chinese, English.
-
Circ_PSD3 Stimulates Cell Proliferation, Migration, Invasion and Epithelial to Mesenchymal Transition (EMT) in Papillary Thyroid Carcinoma via the Regulation of miR-145-5p/miR-338-3p/HMGB3 Axis.J Biochem Mol Toxicol. 2025 Aug;39(8):e70402. doi: 10.1002/jbt.70402. J Biochem Mol Toxicol. 2025. PMID: 40696971
-
A novel long non-coding RNA, PICSAR, promotes thyroid cancer progression through the hsa-miR-320A/hsa-miR-485/RAPGEFL1 axis.Med Oncol. 2025 Aug 26;42(10):448. doi: 10.1007/s12032-025-02987-9. Med Oncol. 2025. PMID: 40856854
-
MicroRNA Expression and Association with Clinicopathologic Features in Papillary Thyroid Cancer: A Systematic Review.Thyroid. 2015 Dec;25(12):1322-9. doi: 10.1089/thy.2015.0193. Epub 2015 Oct 8. Thyroid. 2015. PMID: 26414548
-
Circulating microRNAs and Clinicopathological Findings of Papillary Thyroid Cancer: A Systematic Review.In Vivo. 2022 Jul-Aug;36(4):1551-1569. doi: 10.21873/invivo.12866. In Vivo. 2022. PMID: 35738604 Free PMC article.
References
-
- Rodriguez A., Perron B., Lacroix L., Caillou B., Leblanc G., Schlumberger M., Bidart J., Pourcher T. Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J. Clin. Endocrinol. Metab. 2002;87:3500–3503. doi: 10.1210/jcem.87.7.8797. - DOI - PubMed
-
- Li H., Myeroff L., Smiraglia D., Romero M., Pretlow T., Kasturi L., Lutterbaugh J., Rerko R., Casey G., Issa J., et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc. Natl. Acad. Sci. USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

