Cinnamic Acid: A Shield Against High-Fat-Diet-Induced Liver Injury-Exploring Nrf2's Protective Mechanisms
- PMID: 40869259
- PMCID: PMC12386455
- DOI: 10.3390/ijms26167940
Cinnamic Acid: A Shield Against High-Fat-Diet-Induced Liver Injury-Exploring Nrf2's Protective Mechanisms
Abstract
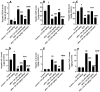
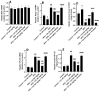
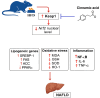
This study investigated the hepatoprotective effects of cinnamic acid (CA) against liver injury and fat accumulation induced by a high-fat diet (HFD), focusing on the role of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling. Male Wistar rats were divided into six groups: a control group receiving carboxymethylcellulose; a CA control group (40 mg/kg); an HFD group; two HFD groups treated with CA (20 mg/kg or 40 mg/kg); and a HFD group co-treated with CA (40 mg/kg) and brusatol (2 mg/kg, i.p.), a selective Nrf2 inhibitor. CA was administered orally, and brusatol intraperitoneally, both twice per week for twelve weeks. CA had no effect on serum glucose or insulin but improved serum and hepatic profiles in HFD rats. It also attenuated liver vacuolization and normalized serum levels of ALT, AST, and γ-GT. CA also reduced hepatic apoptosis by increasing Bcl2 and reducing Bax and caspase-3 levels. CA mitigated oxidative stress by reducing MDA and enhancing SOD and GSH levels. It suppressed inflammatory mediators, including TNF-α, IL-6, and NF-κB. CA also downregulated SREBP1, FAS, ACC-1, and Keap1 while increasing mRNA and nuclear translocation of Nrf2. All these effects were dose-dependent. Similar molecular effects of CA were also seen in control rats while CA protection in HFD rats was abolished with brusatol indicating Nrf2-dependency. Such findings highlight CA as a promising nutraceutical candidate for preventing HFD-induced liver injury. Further studies are warranted to explore its clinical applicability in metabolic liver diseases.
Keywords: Nrf2; cinnamic acid; hepatoprotection; high-fat diet; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Almubark R.A., Alqahtani S., Isnani A.C., Alqarni A., Shams M., Yahia M., Alfadda A.A. Gender differences in the attitudes and management of people with obesity in Saudi Arabia: Data from the ACTION-IO study. Risk Manag. Healthc. Policy. 2022;15:1179–1188. doi: 10.2147/RMHP.S346206. - DOI - PMC - PubMed
-
- Pouwels S., Sakran N., Graham Y., Leal A., Pintar T., Yang W., Kassir R., Singhal R., Mahawar K., Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022;22:63. doi: 10.1186/s12902-022-00980-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

