Preliminary Preclinical Evaluation of Innovative Bone Scaffolds Composed of Natural Sources-Whey Protein Isolate and Pearl Powder
- PMID: 40869260
- PMCID: PMC12386570
- DOI: 10.3390/ijms26167939
Preliminary Preclinical Evaluation of Innovative Bone Scaffolds Composed of Natural Sources-Whey Protein Isolate and Pearl Powder
Abstract
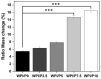
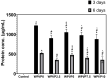
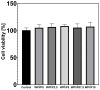
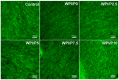
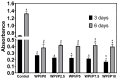
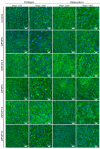
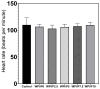
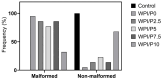
The aim of this work was to produce bone scaffolds containing whey protein isolate and pearl powder and to conduct a preliminary assessment of the biomedical potential in vitro and in vivo. This included analysis of structural, physicochemical, mechanical, and biological properties, which revealed that biomaterials containing pearl powder exhibited an enhanced porous structure, increasing absorptive properties, and decreasing proteolytic capacity with increasing inorganic component content. Pearl powder content in the biomaterials did not clearly influence their mechanical properties or their ability to release calcium ions, as well as proteins. Extracts obtained from all tested biomaterials showed no cytotoxicity in vitro. The surfaces of all biomaterials promoted normal human osteoblast growth, proliferation, and osteogenic differentiation. Furthermore, all biomaterials did not display toxicity in vivo, but no changes in Danio rerio were observed after evaluation of the biomaterial containing the highest amount of pearl powder-10% v/w (marked as WPI/P10). Taking all the obtained results into account, it appears that this biomaterial can be promising for bone scaffolds and similar applications, thanks to its porous structure, high cytocompatibility in vitro, and lack of toxicity in vivo. However, advanced studies will be conducted in the future.
Keywords: Danio rerio; bone; cell-biomaterial interactions; pearl powder; scaffold; whey protein isolate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








Similar articles
-
Exploring mechanisms underlying the beneficial effect of whey protein isolate on the biocompatibility of the thermally obtained curdlan-based hydrogel.Carbohydr Polym. 2025 Oct 15;366:123821. doi: 10.1016/j.carbpol.2025.123821. Epub 2025 May 31. Carbohydr Polym. 2025. PMID: 40733777
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
In vitro characterization of 3D printed polycaprolactone/graphene oxide scaffolds impregnated with alginate and gelatin hydrogels for bone tissue engineering.J Biomater Appl. 2025 Sep;40(3):374-388. doi: 10.1177/08853282251336552. Epub 2025 Apr 25. J Biomater Appl. 2025. PMID: 40278887
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Advancements in Bone Tissue Engineering: A Comprehensive Review of Biomaterial Scaffolds and Freeze-Drying Techniques From Perspective Global and Future Research.Artif Organs. 2025 Aug;49(8):1236-1248. doi: 10.1111/aor.14976. Epub 2025 Feb 24. Artif Organs. 2025. PMID: 39989325
References
-
- Lee S.S., Du X., Kim I., Ferguson S.J. Scaffolds for bone-tissue engineering. Matter. 2022;5:2722–2759. doi: 10.1016/j.matt.2022.06.003. - DOI
-
- Wu A.M., Bisignano C., James S.L., Abady G.G., Abedi A., Abu-Gharbieh E., Alhassan R.K., Alipour V., Arabloo J., Asaad M., et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Heal. Longev. 2021;2:e580–e592. doi: 10.1016/S2666-7568(21)00172-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

