25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells
- PMID: 40869333
- PMCID: PMC12386542
- DOI: 10.3390/ijms26168012
25-Hydroxycholesterol Induces Intrinsic Apoptosis via Mitochondrial Pathway in BE(2)-C Human Neuroblastoma Cells
Abstract
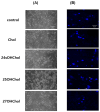
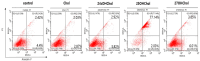
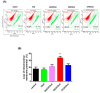
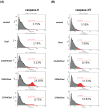
An oxysterol, 25-Hydroxycholesterol (25OHChol), is produced through cholesterol oxidation and is involved in various cellular processes, including apoptosis. However, the precise mechanisms underlying 25OHChol-induced apoptosis in neuroblastoma cells remain unclear. The aim of this study was to elucidate the detailed molecular mechanisms by which 25OHChol induces apoptosis in human neuroblastoma cells. This study explores the apoptotic effects of 25OHChol and the associated signaling pathways in BE(2)-C cells, a widely used human neuroblastoma cell model for neuronal differentiation and cancer research. To evaluate the cytotoxicity of 25OHChol, cell viability was assessed using the CCK-8 assay, which demonstrated a concentration-dependent decline, indicating a potential induction of cell death. Morphological changes characteristic of apoptosis, such as nuclear condensation and fragmentation, were confirmed via DAPI staining. Additionally, Annexin V/PI flow cytometry analysis revealed an increase in late apoptotic cell populations, further corroborating apoptosis induction. To investigate the molecular mechanisms, we analyzed the expression of Bcl-2 family proteins via Western blotting. The results showed an elevated Bax/Bcl-2 ratio, suggesting activation of the intrinsic mitochondrial apoptotic pathway. This was further supported by a reduction in mitochondrial membrane potential (MMP), as measured by flow cytometry. Increased caspase-9 and caspase-3/7 activity provided additional evidence for caspase-mediated apoptosis. Moreover, treatment with the pan-caspase inhibitor Z-VAD-FMK led to a dose-dependent increase in cell viability, confirming the essential role of caspases in 25OHChol-induced apoptosis. In conclusion, this study demonstrates that 25OHChol triggers apoptosis in BE(2)-C neuroblastoma cells through activation of the intrinsic mitochondrial apoptotic pathway. These findings provide new insights into the cytotoxic effects of 25OHChol and its potential role in neuroblastoma cell death.
Keywords: 25-hydroxycholesterol; mitochondria; neuro-inflammation; neuronal cell death; oxysterol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

