Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen
- PMID: 40869346
- PMCID: PMC12386735
- DOI: 10.3390/ijms26168023
Prostaglandins Regulate Urinary Purines by Modulating Soluble Nucleotidase Release in the Bladder Lumen
Abstract
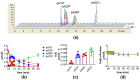
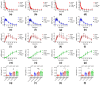
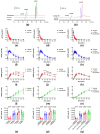
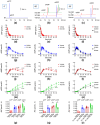
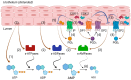
Distention of the urinary bladder wall during filling stretches the urothelium and induces the release of chemical mediators, including adenosine 5'-triphosphate (ATP) and prostaglandins (PGs), that transmit signals between cells within the bladder wall. The urothelium also releases soluble nucleotidases (s-NTDs) that control the availability of ATP and its metabolites at receptor sites in umbrella cells and cells deeper in the bladder wall, as well as in the urine. This study investigated whether PGs regulate the intravesical breakdown of ATP by s-NTDs. Using a murine decentralized mucosa-only bladder model and an HPLC technology with fluorescence detection, we evaluated the decrease in ATP and increase in ADP, AMP, and adenosine (ADO) in intraluminal solutions (ILS) collected at the end of physiological bladder filling. PGD2, PGE2, and PGI2, but not PGF2α, inhibited the conversion of AMP (produced from ATP) to ADO, likely due to a suppressed intravesical release of s-AMPases. The effects of exogenous PGD2, PGE2, and PGI2 were mediated by DP1/DP2, EP2, and IP prostanoid receptors, respectively. Activation of either DP1 or DP2 receptors by endogenous PGD2 also led to AMP increase and ADO decrease in ILS-containing ATP substrate. Finally, PGs produced by either COX-1 or COX-2 inhibited the hydrolysis of AMP to ADO. Together, these observations suggest that (1) endogenous PGs (chiefly PGD2, and to lesser degree PGE2 and PGI2) allow release of s-NTDs like s-ATPases and s-ADPases but impede the formation of ADO from intravesical ATP by inhibiting the release of s-NTDs/s-AMPases; (2) it is possible that high concentrations of PGD2, PGE2 and PGI2, as anticipated in inflammation or bladder pain syndrome, delay the ADO production and prolong the action of excitatory purine mediators; and (3) either COX-1 and COX-2 are constitutively expressed in the mouse bladder mucosa or COX-2 is induced by distention of the urothelium during bladder filling.
Keywords: ATP; bladder; nucleotidases; prostaglandin D2; prostaglandin E2; prostaglandin I2; prostaglandins; urothelium.
Conflict of interest statement
The authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Prostaglandins Differentially Regulate the Constitutive and Mechanosensitive Release of Soluble Nucleotidases in the Urinary Bladder Mucosa.Int J Mol Sci. 2024 Dec 27;26(1):131. doi: 10.3390/ijms26010131. Int J Mol Sci. 2024. PMID: 39795990 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Mechanosensitive release of ATP in the urinary bladder mucosa.Purinergic Signal. 2025 Jun;21(3):413-428. doi: 10.1007/s11302-024-10063-6. Epub 2024 Nov 14. Purinergic Signal. 2025. PMID: 39541058 Free PMC article. Review.
-
Urothelial purine release during filling of murine and primate bladders.Am J Physiol Renal Physiol. 2016 Oct 1;311(4):F708-F716. doi: 10.1152/ajprenal.00387.2016. Epub 2016 Jul 27. Am J Physiol Renal Physiol. 2016. PMID: 27465992 Free PMC article.
-
Lumbar sympathectomy versus prostanoids for critical limb ischaemia due to non-reconstructable peripheral arterial disease.Cochrane Database Syst Rev. 2018 Apr 16;4(4):CD009366. doi: 10.1002/14651858.CD009366.pub2. Cochrane Database Syst Rev. 2018. PMID: 29658630 Free PMC article.
References
-
- Aresta Branco M.S.L., Gutierrez Cruz A., Dayton J., Perrino B.A., Mutafova-Yambolieva V.N. Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases. Front. Physiol. 2022;13:918100. doi: 10.3389/fphys.2022.918100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

