Cannabigerol Attenuates Memory Impairments, Neurodegeneration, and Neuroinflammation Caused by Transient Global Cerebral Ischemia in Mice
- PMID: 40869376
- PMCID: PMC12386769
- DOI: 10.3390/ijms26168056
Cannabigerol Attenuates Memory Impairments, Neurodegeneration, and Neuroinflammation Caused by Transient Global Cerebral Ischemia in Mice
Abstract
Evidence supporting the clinical use of neuroprotective drugs for cerebral ischemia remains limited. Spatial and temporal disorientation, along with cognitive dysfunction, are among the most prominent long-term consequences of hippocampal neurodegeneration following cerebral ischemia. Cannabigerol (CBG), a non-psychotomimetic constituent of Cannabis sativa, has demonstrated neuroprotective effects in experimental models of cerebral injury. This study investigated the neuroprotective mechanisms of CBG in mitigating memory impairments caused by transient global cerebral ischemia in C57BL/6 mice using the bilateral common carotid artery occlusion (BCCAO) model. Mice underwent sham or BCCAO surgeries and received intraperitoneal (i.p.) injections of either a vehicle or CBG (1, 5, or 10 mg/Kg), starting 1 h post-surgery and continuing daily for 7 days. Spatial memory performance and depression-like behaviors were assessed using the object location test (OLT) and tail suspension test (TST), respectively. Additional analyses examined neuronal degeneration, neuroinflammation, and neuronal plasticity markers in the hippocampus. CBG attenuated ischemia-induced memory deficits, reduced neuronal loss in the hippocampus, and enhanced neuronal plasticity. These findings suggest that CBG's neuroprotective effects against BCCAO-induced memory impairments may be mediated by reductions in neuroinflammation and modifications in neuroplasticity within the hippocampus.
Keywords: bilateral common carotid artery occlusion; cannabigerol; cognition; glial response; neuroprotection.
Conflict of interest statement
There are no conflicts of interest in this study.
Figures
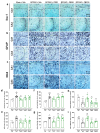
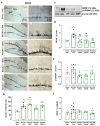


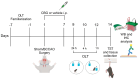
References
-
- Calapai F., Cardia L., Esposito E., Ammendolia I., Mondello C., Lo Giudice R., Gangemi S., Calapai G., Mannucci C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat. Med. 2022;2022:3336516. doi: 10.1155/2022/3336516. - DOI - PMC - PubMed
-
- Deiana S., Watanabe A., Yamasaki Y., Amada N., Arthur M., Fleming S., Woodcock H., Dorward P., Pigliacampo B., Close S., et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (CBD), Cannabidivarine (CBDV), Δ9-Tetrahydrocannabivarin (THCV) and Cannabigerol (CBG) in Rats and Mice Following Oral and Intraperitoneal Administration and CBD Action on Obsessive-Compulsive Behaviour. Psychopharmacology. 2012;219:859–873. doi: 10.1007/s00213-011-2415-0. - DOI - PubMed
-
- di Giacomo V., Chiavaroli A., Recinella L., Orlando G., Cataldi A., Rapino M., Di Valerio V., Ronci M., Leone S., Brunetti L., et al. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020;21:3575. doi: 10.3390/ijms21103575. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

