Immune Modulation Through KIR-HLA Interactions Influences Cetuximab Efficacy in Colorectal Cancer
- PMID: 40869384
- PMCID: PMC12386678
- DOI: 10.3390/ijms26168062
Immune Modulation Through KIR-HLA Interactions Influences Cetuximab Efficacy in Colorectal Cancer
Abstract
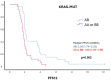
Colorectal cancer (CRC) remains a major cause of cancer-related mortality. Cetuximab improves survival by combining EGFR inhibition with immune activation. This study evaluated the influence of killer cell immunoglobulin-like receptor (KIR)-mediated immune responses on cetuximab efficacy in 124 metastatic CRC patients: 55 with wild-type (WT) KRAS and 69 with KRAS mutations. Peripheral blood was genotyped for 19 KIR genes and relevant HLA alleles, focusing on key KIR-HLA interactions (2DL1-C2, 3DL1-Bw4, 3DS1-Bw4). KRAS-WT patients showed better outcomes, receiving more treatment cycles (median: 17 vs. 4) and showing slower disease progression (60% vs. 92.8% at 12 months). WT patients had higher frequencies of inhibitory KIRs and the Bw4 allele, with KIR3DS1-Bw4 heterozygosity linked to longer survival (p = 0.013). In KRAS-mutant patients, heterozygous KIR genotypes (AB) and mixed A/B semi-haplotypes were associated with improved survival (p = 0.002). Multivariate analysis confirmed KIR3DS1-Bw4 as a favorable factor in WT patients and AB genotypes as beneficial in KRAS-mutants. In conclusion, KIR-HLA interactions significantly impact cetuximab efficacy in metastatic CRC, with distinct immunogenetic profiles in WT and KRAS-mutant patients. These results highlight the potential of KIR-HLA profiling to guide personalized treatment strategies.
Keywords: HLA ligands; KIR; anti-EGFR; cetuximab; metastatic colorectal cancer; natural killer cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
EGFR gene gain and PTEN protein expression are favorable prognostic factors in patients with KRAS wild-type metastatic colorectal cancer treated with cetuximab.J Cancer Res Clin Oncol. 2014 May;140(5):737-48. doi: 10.1007/s00432-014-1626-2. Epub 2014 Mar 5. J Cancer Res Clin Oncol. 2014. PMID: 24595598 Free PMC article.
-
Improving selection of patients with metastatic colorectal cancer to benefit from cetuximab based on KIR genotypes.J Immunother Cancer. 2021 Apr;9(4):e001705. doi: 10.1136/jitc-2020-001705. J Immunother Cancer. 2021. PMID: 33833048 Free PMC article. Clinical Trial.
-
Adagrasib in the treatment of colorectal cancer.Future Oncol. 2025 Aug;21(18):2275-2285. doi: 10.1080/14796694.2025.2524311. Epub 2025 Jul 6. Future Oncol. 2025. PMID: 40619745 Free PMC article. Review.
-
Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.Cochrane Database Syst Rev. 2017 Jun 27;6(6):CD007047. doi: 10.1002/14651858.CD007047.pub2. Cochrane Database Syst Rev. 2017. PMID: 28654140 Free PMC article.
-
The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model.Health Technol Assess. 2013 Apr;17(14):1-237. doi: 10.3310/hta17140. Health Technol Assess. 2013. PMID: 23547747 Free PMC article.
References
-
- Cervantes A., Adam R., Roselló S., Arnold D., Normanno N., Taïeb J., Seligmann J., De Baere T., Osterlund P., Yoshino T., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023;34:10–32. doi: 10.1016/j.annonc.2022.10.003. - DOI - PubMed
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aguilar E.A., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

