Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications
- PMID: 40869425
- PMCID: PMC12386566
- DOI: 10.3390/ijms26168103
Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications
Abstract
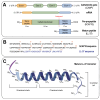
Cathelicidins are a family of antimicrobial peptides (AMPs) with broad-spectrum activity and immunomodulatory functions. Among them, the only human cathelicidin LL-37 has garnered significant interest due to its potent antimicrobial, antiviral, antifungal, antiparasitic, and antitumor properties. However, the clinical application of LL-37 is hindered by several limitations, including low proteolytic stability, cytotoxicity, and high production costs. To overcome these challenges, a wide range of design strategies have been employed to modify LL-37 and improve its therapeutic potential. LL-37-based analogs represent promising candidates for the development of next-generation antimicrobial and immunomodulatory therapies. Despite significant progress, further research is required to optimize peptide design, ensure cost-effective production, and validate long-term safety and efficacy. Advances in computational modeling, high-throughput screening, and nanotechnology will play an important role in the translation of modified cathelicidins into clinical practice. This review summarizes key strategies of chemical and structural modifications of LL-37 aimed at enhancing its functional properties. Particular attention is given to truncated and retro-analogs, which preserve or improve biological activity while exhibiting reduced toxicity and increased proteolytic resistance. Furthermore, we highlight the use of nanoscale delivery systems, which facilitate targeted delivery, prolong peptide half-life, and mitigate cytotoxic effects.
Keywords: LL-37; LL-37 analog design; antimicrobial peptides; cathelicidins; peptide engineering; peptide modification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Fragments of cathelicidins PMAP-36 and BMAP-27 and their D-enantiomers: Effects of all D substitutions on structure, protease resistance and antimicrobial properties.Bioorg Chem. 2025 Aug;163:108715. doi: 10.1016/j.bioorg.2025.108715. Epub 2025 Jun 30. Bioorg Chem. 2025. PMID: 40609280
-
Impact of lipidation site on the activity of α-helical antimicrobial peptides.Bioorg Chem. 2024 Dec;153:107821. doi: 10.1016/j.bioorg.2024.107821. Epub 2024 Sep 13. Bioorg Chem. 2024. PMID: 39293303
-
AI-Driven Antimicrobial Peptide Discovery: Mining and Generation.Acc Chem Res. 2025 Jun 17;58(12):1831-1846. doi: 10.1021/acs.accounts.0c00594. Epub 2025 Jun 3. Acc Chem Res. 2025. PMID: 40459283 Free PMC article. Review.
-
Production, Delivery, and Regulatory Aspects for Application of Plant-Based Anti-microbial Peptides: a Comprehensive Review.Probiotics Antimicrob Proteins. 2025 Aug;17(4):2362-2393. doi: 10.1007/s12602-024-10421-1. Epub 2025 Jan 4. Probiotics Antimicrob Proteins. 2025. PMID: 39753941 Review.
References
-
- Naghavi M., Vollset S.E., Ikuta K.S., Swetschinski L.R., Gray A.P., Wool E.E., Robles Aguilar G., Mestrovic T., Smith G., Han C., et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet. 2024;404:1199–1226. doi: 10.1016/S0140-6736(24)01867-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

