Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol
- PMID: 40869727
- PMCID: PMC12387123
- DOI: 10.3390/jcm14165901
Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol
Abstract
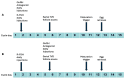
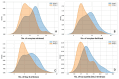
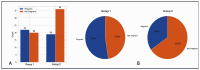
Objectives: Gonadotropin-releasing hormone (GnRH) antagonist protocols are preferred in polycystic ovary syndrome (PCOS) patients undergoing in vitro fertilization (IVF) as they provide the best combination of flexibility, acceptable outcomes, and safety. Numerous studies have compared outcomes between GnRH agonist long protocol and standard flexible antagonist protocol. However, there are scant studies investigating the effectiveness of antagonist administration from day 1 of ovarian stimulation in PCOS patients. Methods: We performed a retrospective cohort study to compare laboratory and clinical outcomes in IVF between standard flexible day 5/day 6 versus day 1 GnRH antagonist protocol in PCOS patients. Results: Our data indicates significantly superior oocyte yield and top-quality embryo proportion in patients with antagonists from day 1. Cumulative clinical pregnancy rates also tended to be superior in this group. Conclusions: Our findings indicate that administration of GnRH antagonists from day 1 of stimulation in PCOS patients undergoing IVF may lead to superior results.
Keywords: GnRH antagonist; in vitro fertilization (IVF) outcome; luteinizing hormone (LH); ovarian stimulation (OS); pituitary suppression; polycystic ovarian syndrome (PCOS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Balen A.H., Morley L.C., Misso M., Franks S., Legro R.S., Wijeyaratne C.N., Stener-Victorin E., Fauser B.C., Norman R.J., Teede H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update. 2016;22:687–708. doi: 10.1093/humupd/dmw025. - DOI - PubMed
-
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Futterweit W., Janssen O.E., Legro R.S., Norman R., Taylor A.E., et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. - DOI - PubMed
LinkOut - more resources
Full Text Sources

