Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton
- PMID: 40870015
- PMCID: PMC12385653
- DOI: 10.3390/genes16080968
Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton
Abstract
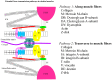
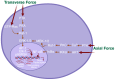
Mechanotransduction, also referred to as mechano-signal transduction, is a biophysical process wherein cells perceive and respond to mechanical stimuli by converting them into biochemical signals that initiate specific cellular responses. This mechanism is fundamental to the development and growth, and proper functioning of mechanically active tissues, such as the diaphragm-a respiratory muscle vital for breathing in mammals. In vivo, the diaphragm is subjected to transdiaphragmatic pressure, and therefore, its muscle fibers are subjected to mechanical forces not only in the direction of the muscle fibers but also in the direction transverse to the fibers. Previous research conducted in our laboratory uncovered that stretching the diaphragm in either the longitudinal or transverse direction activates distinct mechanotransduction pathways. This indicates that signaling pathways in the diaphragm muscle are regulated in an anisotropic manner. In this review paper, we discussed the underlying mechanisms that regulate the anisotropic signaling pathways in the diaphragmatic muscle, emphasizing the mechanical role of cytoskeletal proteins in this context. Furthermore, we explored the regulatory mechanisms governing mechanosensitive gene transcription, including microRNAs (mechanomiRs), within the diaphragm muscle. Finally, we examined potential links between anisotropic signaling in the diaphragm muscle and various skeletal muscle disorders.
Keywords: anisotropic gene regulation; cytoskeletal proteins; mechanical stretch; mechanomiRs; mechanotransduction; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy.Cell Commun Signal. 2024 Mar 27;22(1):197. doi: 10.1186/s12964-024-01546-5. Cell Commun Signal. 2024. PMID: 38539233 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Characterization of SARS-CoV-2 Entry Genes in Skeletal Muscle and Impacts of In Vitro Versus In Vivo Infection.J Cachexia Sarcopenia Muscle. 2025 Feb;16(1):e13705. doi: 10.1002/jcsm.13705. J Cachexia Sarcopenia Muscle. 2025. PMID: 39871399 Free PMC article.
-
Prenatal interventions for congenital diaphragmatic hernia for improving outcomes.Cochrane Database Syst Rev. 2015 Nov 27;2015(11):CD008925. doi: 10.1002/14651858.CD008925.pub2. Cochrane Database Syst Rev. 2015. PMID: 26611822 Free PMC article.
References
-
- Miyasaka K., Kida Y., Ogura T. Mechanotransduction in cardiovascular and skeletal muscle. Seikagaku. 2009;81:494–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

