Identification of a Pathogenic Mutation for Glycogen Storage Disease Type II (Pompe Disease) in Japanese Quails (Coturnix japonica)
- PMID: 40870023
- PMCID: PMC12386088
- DOI: 10.3390/genes16080975
Identification of a Pathogenic Mutation for Glycogen Storage Disease Type II (Pompe Disease) in Japanese Quails (Coturnix japonica)
Abstract
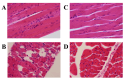
Background/Objectives: Pompe disease (PD) is a rare autosomal recessive disorder caused by a deficiency of the lysosomal acid α-1,4-glucosidase (GAA) encoded by the GAA gene, leading to muscular dysfunctions due to pathological accumulation of glycogen in skeletal and cardiac muscles. PD has been reported in several animals and Japanese quails (JQ; Coturnix japonica), but a causative mutation has yet to be found in JQs with PD. Here, we aimed to identify a pathogenic mutation in JQs associated with PD. Methods: Paraffin-embedded skeletal muscle blocks from four JQs stored since the 1970s were used in this study. After confirming the histopathological phenotypes of PD, Sanger sequencing was performed to identify a pathological mutation in the GAA I gene of JQs. A genotyping survey was conducted using a real-time polymerase chain reaction assay targeting a candidate mutation using DNA samples extracted from 70 new-hatched JQs and 10 eggs from commercial farms. Results: Microscopic analysis confirmed the presence of the PD phenotype in three affected JQs based on abnormal histopathological changes and accumulated glycogen in the affected muscles, while one JQ was unaffected and served as a control. Sanger sequencing revealed that the three affected JQs were homozygous for the deletion of guanine at position 1096 in the open reading frame (c.1096delG). A genotyping survey of 70 JQs and 10 eggs from commercial farms showed that none carried this deletion mutation. Conclusions: This study identified c.1096delG as the pathogenic mutation for PD in JQs. This mutation induces a frameshift and substitution of amino acids at position 366 (alanine to histidine), resulting in premature termination at the 23rd codon (p.A366Hfs*23). This suggests that this mutation causes the deficient activity of GAA in JQs with PD. The identification of the c.1096delG mutation enabled the systematic maintenance of the flock colony in the PD model. Furthermore, this PD model can be used to clarify unknown aspects of PD pathogenesis and develop therapeutic strategies.
Keywords: GAA I gene; Japanese quail; Pompe disease; deletion mutation; glycogen storage disease type II.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- OMIM #232300, Pompe Disease. [(accessed on 25 July 2025)]. Available online: https://omim.org/entry/232300.
-
- Niño M.Y., In’t Groen S.L., Bergsma A.J., van der Beek N.A., Kroos M., Hoogeveen-Westerveld M., van der Ploeg A.T., Pijnappel W.P. Extension of the Pompe mutation database by linking disease-associated variants to clinical severity. Hum. Mutat. 2019;40:1954–1967. doi: 10.1002/humu.23854. - DOI - PMC - PubMed
-
- Pompe Disease GAA Variant Database. [(accessed on 25 July 2025)]. Available online: https://www.pompevariantdatabase.clmz.nl/pompe_mutations_list.php?orderb....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

