Diagnostic and Clinical Outcomes of Three Regenerative Strategies for Alveolar Bone Defects: A Comparative Study Using CBCT and ISQ
- PMID: 40870931
- PMCID: PMC12385923
- DOI: 10.3390/diagnostics15162078
Diagnostic and Clinical Outcomes of Three Regenerative Strategies for Alveolar Bone Defects: A Comparative Study Using CBCT and ISQ
Abstract
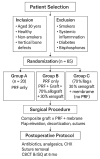
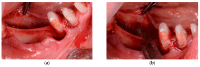
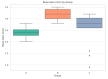
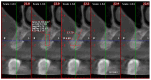
Background: This prospective clinical study aimed to evaluate the effectiveness of platelet-rich fibrin (PRF) in guided bone regeneration (GBR) prior to dental implant placement. Material and methods: Sixty-five patients with alveolar bone defects were randomly assigned to three groups. All groups received a composite graft consisting of 70% allograft and 30% xenograft. Group A received the graft combined with PRF. Group B received the graft with PRF and a resorbable collagen membrane. Group C (control) received the same graft and membrane without PRF. Cone-beam computed tomography (CBCT) was used to assess bone regeneration at baseline and 6 months postoperatively. Implant stability was evaluated using ISQ values at the time of implant placement (6 months after grafting) and again at 3 to 4 months during the second-stage uncovering procedure. Soft tissue healing, postoperative complications, and pain scores were also recorded. Results: Group B showed the best outcomes, with the highest mean vertical bone gain (3.0 ± 0.4 mm), greatest implant stability (ISQ: 74.2 ± 1.8), and no complications. Group A achieved moderate bone gain (2.3 ± 0.4 mm) and good ISQ values (71.5 ± 2.3), with favorable soft tissue healing. In contrast, Group C had the lowest bone gain (2.1 ± 0.5 mm), reduced ISQ values (68.9 ± 2.9), and the highest incidence of complications, including dehiscence and minor infections. Conclusions: These results suggest that PRF enhances both hard and soft tissue regeneration, particularly when used with grafts and membranes. PRF may reduce healing time and postoperative discomfort, improving the overall success of regenerative implant procedures.
Keywords: bone graft; collagen membrane; guided bone regeneration; implant stability; platelet-rich fibrin; soft tissue healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
Horizontal ridge augmentation using guided bone regeneration with an association of particulate allografts mixed with platelet-rich fibrin, collagen membrane and tent-screws: A prospective study.J Stomatol Oral Maxillofac Surg. 2024 Sep;125(12 Suppl 2):101872. doi: 10.1016/j.jormas.2024.101872. Epub 2024 Apr 4. J Stomatol Oral Maxillofac Surg. 2024. PMID: 38582352
-
A novel polycaprolactone mesh for buccal bone augmentation with simultaneous implant placement: A feasibility report of three cases.Clin Adv Periodontics. 2025 Aug 21. doi: 10.1002/cap.70007. Online ahead of print. Clin Adv Periodontics. 2025. PMID: 40838266
-
Evaluation of Effectiveness of Nanocrystalline Hydroxyapatite and Demineralized Bone Matrix Combined with Titanium-platelet Rich Fibrin for Ridge Preservation: A Randomized Controlled Clinical Trial.J Contemp Dent Pract. 2024 Nov 1;25(11):1069-1076. doi: 10.5005/jp-journals-10024-3786. J Contemp Dent Pract. 2024. PMID: 39905614 Clinical Trial.
-
Guided tissue regeneration for periodontal infra-bony defects.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD001724. doi: 10.1002/14651858.CD001724.pub3. PMID: 16625546 Updated.
-
Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus.Cochrane Database Syst Rev. 2014 May 13;2014(5):CD008397. doi: 10.1002/14651858.CD008397.pub2. Cochrane Database Syst Rev. 2014. PMID: 24825543 Free PMC article.
References
-
- Aghaloo T.L., Moy P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implant. 2007;22:49–70. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

