Advancements in Diagnosis of Neoplastic and Inflammatory Skin Diseases: Old and Emerging Approaches
- PMID: 40870951
- PMCID: PMC12385878
- DOI: 10.3390/diagnostics15162100
Advancements in Diagnosis of Neoplastic and Inflammatory Skin Diseases: Old and Emerging Approaches
Abstract
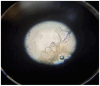
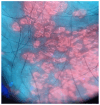
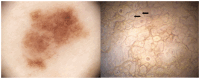
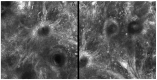
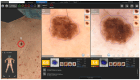
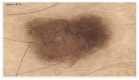
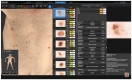
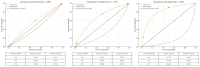
Background: In recent decades, dermatological diagnostics have undergone a profound transformation, driven by the integration of new technologies alongside traditional methods. Classic techniques such as the Tzanck smear, potassium hydroxide (KOH) preparation, and Wood's lamp examination remain fundamental in everyday clinical practice due to their simplicity, speed, and accessibility. At the same time, the development of non-invasive imaging technologies and the application of artificial intelligence (AI) have opened new frontiers in the early detection and monitoring of both neoplastic and inflammatory skin diseases. Methods: This review aims to provide a comprehensive overview of how conventional and emerging diagnostic tools can be integrated into dermatologic practice. Results: We examined a broad spectrum of diagnostic methods currently used in dermatology, ranging from traditional techniques to advanced approaches such as digital dermoscopy, reflectance confocal microscopy (RCM), optical coherence tomography (OCT), line-field confocal OCT (LC-OCT), 3D total body imaging systems with AI integration, mobile applications, electrical impedance spectroscopy (EIS), and multispectral imaging. Each method is discussed in terms of diagnostic accuracy, clinical applications, and potential limitations. While traditional methods continue to play a crucial role-especially in resource-limited settings or for immediate bedside decision-making-modern tools significantly enhance diagnostic precision. Dermoscopy and its digital evolution have improved the accuracy of melanoma and basal cell carcinoma detection. RCM and LC-OCT allow near-histological visualization of skin structures, reducing the need for invasive procedures. AI-powered platforms support lesion tracking and risk stratification, though their routine implementation requires further clinical validation and regulatory oversight. Tools like EIS and multispectral imaging may offer additional value in diagnostically challenging cases. An effective diagnostic approach in dermatology should rely on a thoughtful combination of methods, selected based on clinical suspicion and guided by Bayesian reasoning. Conclusions: Rather than replacing traditional tools, advanced technologies should complement them-optimizing diagnostic accuracy, improving patient outcomes, and supporting more individualized, evidence-based care.
Keywords: artificial intelligence; confocal microscopy; dermatologic diagnosis; dermoscopy; digital health; inflammatory skin diseases; line-field OCT; skin cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Forsea A.M., Tschandl P., Zalaudek I., Del Marmol V., Soyer H.P., Eurodermoscopy Working Group. Argenziano G., Geller A.C. The impact of dermoscopy on melanoma detection in the practice of dermatologists in Europe: Results of a pan-European survey. J. Eur. Acad. Dermatol. Venereol. 2017;31:1148–1156. doi: 10.1111/jdv.14129. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

