Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions
- PMID: 40871032
- PMCID: PMC12388975
- DOI: 10.3390/pharmaceutics17081010
Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions
Abstract
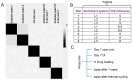
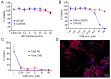
Background: Pain is a complex phenomenon characterized by unpleasant experiences with profound heterogeneity influenced by biological, psychological, and social factors. According to the National Health Interview Survey, 50.2 million U.S. adults (20.5%) experience pain on most days, with the annual cost of prescription medication for pain reaching approximately USD 17.8 billion. Theranostic pain nanomedicine therefore emerges as an attractive analgesic strategy with the potential for increased efficacy, reduced side-effects, and treatment personalization. Theranostic nanomedicine combines drug delivery and diagnostic features, allowing for real-time monitoring of analgesic efficacy in vivo using molecular imaging. However, clinical translation of these nanomedicines are challenging due to complex manufacturing methodologies, lack of standardized quality control, and potentially high costs. Quality by Design (QbD) can navigate these challenges and lead to the development of an optimal pain nanomedicine. Our lab previously reported a macrophage-targeted perfluorocarbon nanoemulsion (PFC NE) that demonstrated analgesic efficacy across multiple rodent pain models in both sexes. Here, we report PFC-free, biphasic nanoemulsions formulated with a biocompatible and non-immunogenic plant-based coconut oil loaded with a COX-2 inhibitor and a clinical-grade, indocyanine green (ICG) near-infrared fluorescent (NIRF) dye for parenteral theranostic analgesic nanomedicine. Methods: Critical process parameters and material attributes were identified through the FMECA (Failure, Modes, Effects, and Criticality Analysis) method and optimized using a 3 × 2 full-factorial design of experiments. We investigated the impact of the oil-to-surfactant ratio (w/w) with three different surfactant systems on the colloidal properties of NE. Small-scale (100 mL) batches were manufactured using sonication and microfluidization, and the final formulation was scaled up to 500 mL with microfluidization. The colloidal stability of NE was assessed using dynamic light scattering (DLS) and drug quantification was conducted through reverse-phase HPLC. An in vitro drug release study was conducted using the dialysis bag method, accompanied by HPLC quantification. The formulation was further evaluated for cell viability, cellular uptake, and COX-2 inhibition in the RAW 264.7 macrophage cell line. Results: Nanoemulsion droplet size increased with a higher oil-to-surfactant ratio (w/w) but was no significant impact by the type of surfactant system used. Thermal cycling and serum stability studies confirmed NE colloidal stability upon exposure to high and low temperatures and biological fluids. We also demonstrated the necessity of a solubilizer for long-term fluorescence stability of ICG. The nanoemulsion showed no cellular toxicity and effectively inhibited PGE2 in activated macrophages. Conclusions: To our knowledge, this is the first instance of a celecoxib-loaded theranostic platform developed using a plant-derived hydrocarbon oil, applying the QbD approach that demonstrated COX-2 inhibition.
Keywords: Quality by Design (QbD); celecoxib; coconut oil; macrophage; nanoemulsion; pain.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Dahlhamer J.M., Connor E.M., Bose J., Lucas J.W., Zelaya C.E. Prescription Opioid Use Among Adults with Chronic Pain: United States, 2019. National Center for Health Statistics; Hyattsville, MD, USA: 2021. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

