CUSP06, a Novel CDH6-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Efficacy in Multiple CDH6-Expressing Human Cancer Models
- PMID: 40871070
- PMCID: PMC12388900
- DOI: 10.3390/pharmaceutics17081049
CUSP06, a Novel CDH6-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Efficacy in Multiple CDH6-Expressing Human Cancer Models
Abstract
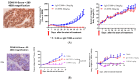
Background/Objectives: Cadherin-6 (CDH6), also known as K-cadherin, is a type II classic cadherin molecule that plays an important role in the embryonic development of the kidney but has very limited expression in adult tissues. It is overexpressed in several human malignancies, primarily in ovarian cancer, renal cell carcinoma, as well as, less frequently, cholangiocarcinoma, uterine serous carcinoma, glioma, lung, pancreatic and thyroid cancers. The characteristic of limited expression in normal tissues, high expression in tumor tissues, and rapid internalization upon antibody binding makes CDH6 a well-suited antibody-drug conjugate (ADC) target. Methods: We developed a novel CDH6-targeting ADC, CUSP06, consisting of a proprietary humanized antibody selective for CDH6, a protease cleavable linker, and an exatecan payload, with a drug-to-antibody ratio (DAR) of 8. We further characterized the pharmacological activities of CUSP06 in multiple in vitro and in vivo models. Results: CUSP06 was selectively bound to cell surface CDH6 and was efficiently internalized into CDH6-positive ovarian cancer cells, and led to the induction of DNA damage and apoptosis of CDH6-positive cancer cells. CUSP06 exhibited strong antiproliferative activity against several CDH6-positive cancer cell lines and demonstrated strong bystander cell killing effect in the cell mixing experiments in vitro. CUSP06 exhibits excellent in vivo antitumor efficacy in CDH6-high or -low cell line-derived xenograft (CDX) or patient-derived xenograft (PDX) models from human ovarian, renal and uterine cancers, as well as cholangiocarcinoma. CUSP06 demonstrated a favorable safety profile in GLP-compliant toxicology studies in Sprague Dawley rats and cynomolgus monkeys. Conclusions: The preclinical data highlighted the therapeutic potential of CUSP06 in multiple CDH6-positive human cancers.
Keywords: ADC (antibody-drug conjugate); CDH6; K-cadherin; cadherin-6; cholangiocarcinoma; exatecan; ovarian cancer; uterine cancer.
Conflict of interest statement
N. Covino, W. Lu, A. Penticoff, E. Slosberg, and W. Zhang are employees and shareholders of OnCusp Therapeutics. J. Cogswell, R. Phillips, S. Paras-Farmer, and L. Tatalick are consultants for OnCusp Therapeutics. S. Pasas-Farmer is the founder and president of BioData Solutions. J. Shi, J. Zhang, C. Chen, Y. Wang, H. Shi, S. Liu, and X. Meng are employees of Multitude Therapeutics; S. Liu and X. Meng are also shareholders of Multitude Therapeutics.
Figures







References
-
- Colombo R., Tarantino P., Rich J.R., LoRusso P.M., De Vries E.G.E. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024;14:2089–2108. doi: 10.1158/2159-8290.CD-24-0708. - DOI - PubMed
-
- Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N., Soma M., Okamoto H., Oitate M., Arakawa S., et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. - DOI - PubMed
LinkOut - more resources
Full Text Sources

