Characterization of a Thermostable α-Amylase from Bacillus licheniformis 104.K for Industrial Applications
- PMID: 40871262
- PMCID: PMC12388735
- DOI: 10.3390/microorganisms13081757
Characterization of a Thermostable α-Amylase from Bacillus licheniformis 104.K for Industrial Applications
Abstract
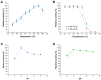
This study describes the characterization of a novel thermostable α-amylase from a Bacillus licheniformis 104.K strain isolated from the Kashkadarya region of Uzbekistan. Phylogenetic analysis revealed that the thermostable α-amylase belongs to glycoside hydrolase family 13 subfamily 5 (GH13_5) and shares high sequence similarity with known α-amylases. Our results demonstrate that the recombinant α-amylase exhibits optimal activity at pH 6.0 and 90 °C, retaining full activity after 30 min at 60 °C. The addition of CaCl2 significantly enhanced thermostability, with the enzyme retaining more than 95% of its initial activity at 70 °C after 30 min. Our findings indicate that α-amylase from B. licheniformis 104.K is a functional, thermostable enzyme with potential industrial applications. This study highlights the commercial significance of thermostable amylases and the need to identify novel, cost-effective, and sustainable sources. The results of this study will contribute to the fields of enzyme applications, stabilizing additives, and genetic engineering of thermostable genes.
Keywords: Bacillus licheniformis; GH13_5 subfamily; affinity chromatography; recombinant enzyme; thermostability; α-amylase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
- IL-4821091620/Agency of Innovative Development of the Republic of Uzbekistan
- 2022-1.2.2-TÉT-IPARI-UZ-2022-00003/National Research, Development and Innovation Office of Hungary
- Bolyai János Research Scholarship to GNN/Hungarian Academy of Sciences
- Somogyi Mihály Grant to GNN/Varga József Foundation, Budapest University of Technology and Economics
LinkOut - more resources
Full Text Sources

