Three-Dimensional Structure of Biofilm Formed on Glass Surfaces Revealed Using Scanning Ion Conductance Microscopy Combined with Confocal Laser Scanning Microscopy
- PMID: 40871283
- PMCID: PMC12388386
- DOI: 10.3390/microorganisms13081779
Three-Dimensional Structure of Biofilm Formed on Glass Surfaces Revealed Using Scanning Ion Conductance Microscopy Combined with Confocal Laser Scanning Microscopy
Abstract
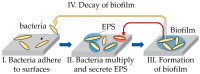
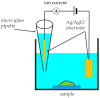
Biofilms cause a variety of problems, such as food spoilage, food poisoning, infection, tooth decay, periodontal disease, and metal corrosion, so knowledge on biofilm prevention and removal is important. A detailed observation of the three-dimensional structure of biofilms on the nanoscale is expected to provide insight into this. In this study, we report on the successful in situ nanoscale observations of a marine bacterial biofilm on glass in phosphate buffer solution (PBS) using both scanning ion conductance microscopy (SICM) and confocal laser scanning microscopy (CLSM) over the same area. By observing the same area by SICM and CLSM, we were able to clarify the three-dimensional morphology of the biofilm, the arrangement of bacteria within the biofilm, and the difference in local ion conductivity within the biofilm simultaneously, which could not be achieved by observation using a microscope alone.
Keywords: Aliivibrio fischeri; biofilm; confocal laser scanning microscope; in situ observation; scanning ion conductive microscope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Park S.-H., Kang D.-H. Influence of surface properties of produce and food contact surfaces on the efficacy of chlorine dioxide gas for the inactivation of foodborne pathogens. Food Control. 2017;81:88–95. doi: 10.1016/j.foodcont.2017.05.015. - DOI
-
- Page K., Wilson M., Mordan N.J., Chrzanowski W., Knowles J., Parkin I.P. Study of the adhesion of Staphylococcus aureus to coated glass substrates. J. Mater. Sci. 2011;46:6355–6363. doi: 10.1007/s10853-011-5582-9. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

