BOL Lectin: A Protein Derived from Cauliflower Exhibits Antibiofilm Activity in In Vitro Assays Against Staphylococcus aureus
- PMID: 40871405
- PMCID: PMC12388089
- DOI: 10.3390/microorganisms13081901
BOL Lectin: A Protein Derived from Cauliflower Exhibits Antibiofilm Activity in In Vitro Assays Against Staphylococcus aureus
Abstract
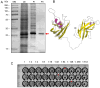
The BOL lectin, a 34 kDa protein with a hemagglutination titer of 64 hemagglutination units (HU), was extracted from cauliflower (Brassica oleracea spp. botrytis L.), purified by affinity and ion exchange chromatography, and confirmed, in this study, by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). The antibiofilm activity of BOL was evaluated at two concentrations (0.1 mg/mL and 1.0 mg/mL) against bacterial strains of importance to human health (Bacillus cereus ATCC 10876, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, and Streptococcus agalactiae ATCC 12403). In addition to a biofilm formation assay, a pre-formed biofilm assay was conducted, with biofilm structure analyzed by Scanning Electron Microscopy (SEM). The antimicrobial potential of BOL was also investigated using the Minimum Inhibitory Concentration (MIC) assay in 96-well microplates. Among the tested bacterial strains, BOL exhibited activity against S. aureus at 1.0 mg/mL, interfering with both biofilm formation and disrupting pre-formed biofilms, which may be explained by a possible interaction between BOL and the components present in the biofilm matrix. However, no antibiofilm activity was observed against E. coli, B. cereus, or S. agalactiae, possibly due to differences in the composition of their biofilm matrices. Furthermore, BOL showed no detectable bactericidal or bacteriostatic activity in the antimicrobial assays. In conclusion, BOL lectin, at the tested concentrations, does not exhibit direct antimicrobial activity but effectively disrupts the extracellular matrix in S. aureus ATCC 29213.
Keywords: One Health; antimicrobial activity; bacterial resistance; biofilms; biotechnology; plant lectin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Procópio T.F., Patriota L.L.d.S., Moura M.C.d., Silva P.M.d., Oliveira A.P.S.d., Carvalho L.V.d.N., Lima T.d.A., Soares T., Silva T.D.d., Coelho L.C.B.B., et al. CasuL: A New Lectin Isolated from Calliandra surinamensis Leaf Pinnulae with Cytotoxicity to Cancer Cells, Antimicrobial Activity and Antibiofilm Effect. Int. J. Biol. Macromol. 2017;98:419–429. doi: 10.1016/j.ijbiomac.2017.02.019. - DOI - PubMed
-
- Almeida Campos L.A.d., Costa Junior S.D.d., Santos J.V.d.O., Souza Z.N.d., Silva C.E.S.d., Cristovão-Silva A.C., Brelaz-de-Castro M.C.A., Pereira V.R.A., Paiva P.M.G., Santos Correia M.T.d., et al. Anti-Staphylococcal, Antibiofilm and Trypanocidal Activities of CrataBL Encapsulated into Liposomes: Lectin with Potential against Infectious Diseases. Microb. Pathog. 2024;196:107007. doi: 10.1016/j.micpath.2024.107007. - DOI - PubMed
-
- Konozy E.H.E., Osman M.E.M., Dirar A.I., Osman R.S.H. Revolutionizing Therapeutics: The Dazzling World of Plant Lectins. J. King Saud. Univ.–Sci. 2024;36:103318. doi: 10.1016/j.jksus.2024.103318. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

