Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives
- PMID: 40871507
- PMCID: PMC12388491
- DOI: 10.3390/molecules30163354
Synthesis and Properties of Silver Nanoparticles Functionalized with β-Cyclodextrin and Their Loading with Lupinine and Its Acetyl Derivatives
Abstract
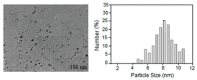
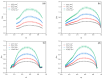
This study presents the results of a study of the synthesis and properties of 2-hydroxy-β-cyclodextrin functionalized by silver nanoparticles and its loading with a bioactive component. As a reducing agent and stabilizer, 2-Hydroxy-β-cyclodextrin (2gβCD) was used in the production of silver nanoparticles. The use of 2gβCD-AgNPs in loading molecules of the plant alkaloid lupinine (Lup) and its acetyl derivative (Lac) with bactericidal properties were studied. The formation of Lup-2gβCD-AgNPs and Lac-2gβCD-AgNPs was confirmed by UV spectroscopy and X-ray diffraction spectroscopy (XRD). Transmission electron microscopy (TEM) showed that the synthesized AgNPs had a spherical shape. 1H-, 13C-NMR nuclear magnetic resonance spectroscopy and Fourier transform infrared spectroscopy (FT-IR) confirmed the reduction and encapsulation of AgNPs by 2gβCD. Thermographic data show that the obtained Lup and its derivative inclusion complexes reduced energy barriers. This makes them promising components for thermosensitive functional materials. Encapsulated complexes of Lup and its acetate inclusion with silver nanoparticles demonstrated significantly (p < 0.05) higher antibacterial, cytotoxic, and moderately pronounced analgesic activity.
Keywords: 2-hydroxypropyl-β-cyclodextrin; analgetic activities; bactericidal; cytotoxic; lupinine; lupinyl acetate; nanocomposite; silver nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








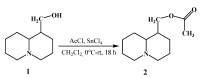

Similar articles
-
Biosynthesis and characterization of silver nanoparticles from Asplenium dalhousiae and their potential biological properties.PLoS One. 2025 Jun 30;20(6):e0325533. doi: 10.1371/journal.pone.0325533. eCollection 2025. PLoS One. 2025. PMID: 40587502 Free PMC article.
-
Characterization of Silver Nanoparticles Synthesized Using Hypericum perforatum L. and Their Effects on Staphylococcus aureus.Microsc Res Tech. 2025 Aug;88(8):2321-2332. doi: 10.1002/jemt.24862. Epub 2025 Mar 23. Microsc Res Tech. 2025. PMID: 40121669 Free PMC article.
-
Structural insights and biomedical potential of biosynthesized silver nanoparticles: antibacterial activity, anti-biofilm and cancer cell inhibition.PeerJ. 2025 Jul 1;13:e19608. doi: 10.7717/peerj.19608. eCollection 2025. PeerJ. 2025. PMID: 40620779 Free PMC article.
-
Phytogenic nanoparticles: synthesis, characterization, and their roles in physiology and biochemistry of plants.Biometals. 2024 Feb;37(1):23-70. doi: 10.1007/s10534-023-00542-5. Epub 2023 Nov 2. Biometals. 2024. PMID: 37914858 Review.
-
Lignin micro-nanospheres loaded with silver nanoparticles for excellent antibacterial activity.Int J Biol Macromol. 2025 Aug;319(Pt 2):145374. doi: 10.1016/j.ijbiomac.2025.145374. Epub 2025 Jun 19. Int J Biol Macromol. 2025. PMID: 40543783 Review.
References
-
- Nurmaganbetov Z.S., Nurkenov O.A., Khlebnikov A.I., Fazylov S.D., Seidakhmetova R.B., Tukhmetova Z.K., Takibayeva A.T., Khabdolda G., Rakhimberlinova Z.B., Kaldybayeva A.K., et al. Antiviral Activity of (1S,9aR)-1-[(1,2,3-Triazol-1-yl)-methyl]-octahydro-1H-quinolizines from the Alkaloid Lupinine. Molecules. 2024;29:5742. doi: 10.3390/molecules29235742. - DOI - PMC - PubMed
-
- Magalhaes S., Fernandes F., Cabrita A.R.J., Fonseca A.J.M., Valentao P., Andrade P.B. Alkaloids in the valorization of European Lupinus spp. seeds crop. Ind. Crops Prod. 2016;95:286–295. doi: 10.1016/j.indcrop.2016.10.033. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

