Efficient Photo-Driven Electron Transfer from Amino Group-Decorated Adamantane to Water
- PMID: 40871549
- PMCID: PMC12388030
- DOI: 10.3390/molecules30163396
Efficient Photo-Driven Electron Transfer from Amino Group-Decorated Adamantane to Water
Abstract
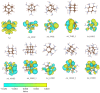
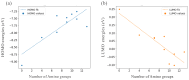
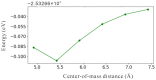
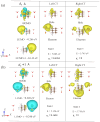
Nanodiamonds in water can generate solvated electrons under ultraviolet (UV) excitation, but UV light constitutes only a small portion of solar energy. To harvest solar energy in the visible range, we investigate band gap reduction via surface amino functionalization and examine its impact on photo-excited charge transfer to water. Adamantane, the smallest nanodiamond, is used as a model due to its electron emission properties. Liquid water is first represented using water dimers and then complete solvation shell structures surrounding the adamantane. By systematically analyzing different functionalized adamantane structures, we find that nitrogen serves as the primary electron donor to nearby water molecules. Furthermore, the negative electron affinity of adamantane, which determines its emission capability, is preserved with half of the amino group functionalization on the surface. Our findings motivate further experimental verification using nanodiamonds with amino-functionalized surfaces.
Keywords: adamantane; amino group; electron transfer; excited states; linear response time-dependent density functional theory (LR-TDDFT).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Friedlingstein P., O’Sullivan M., Jones M.W., Andrew R.M., Hauck J., Olsen A., Peters G.P., Peters W., Pongratz J., Sitch S., et al. Global Carbon Budget 2020. Earth Syst. Sci. Data Discuss. 2020;2020:1–3. doi: 10.5194/essd-12-3269-2020. - DOI
-
- Su L.X., Cao Y., Hao H.S., Zhao Q., Zhi J. Emerging Applications of Nanodiamonds in Photocatalysis. Funct. Diam. 2022;1:93–109. doi: 10.1080/26941112.2020.1869431. - DOI
-
- Hazen R.M., Hemley R.J., Mangum A.J. Carbon in Earth’s Interior: Storage, Cycling, and Life. Eos Trans. Am. Geophys. Union. 2012;93:17–18. doi: 10.1029/2012EO020001. - DOI
-
- Danilenko V.V. On the History of the Discovery of Nanodiamond Synthesis. Phys. Solid State. 2004;46:595–599. doi: 10.1134/1.1711431. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

