Chemical Profiling and UPLC-qTOF-MS/MS-Based Metabolomics of Three Different Parts of Edgeworthia chrysantha and Identification of Glucose Uptake-Enhancing Compounds
- PMID: 40871711
- PMCID: PMC12389323
- DOI: 10.3390/nu17162684
Chemical Profiling and UPLC-qTOF-MS/MS-Based Metabolomics of Three Different Parts of Edgeworthia chrysantha and Identification of Glucose Uptake-Enhancing Compounds
Abstract
Background/objectives: Edgeworthia chrysantha is rich in coumarin and flavonoid dimers, which may exhibit diverse pharmacological activities. However, to date, no metabolomics studies have been conducted and its bioactive constituents related to glucose metabolism remain uncharacterized. This study aimed to conduct a comprehensive chemical analysis combined with bioactivity assays to evaluate its efficacy in promoting glucose uptake.
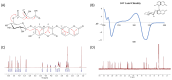
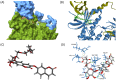
Methods: Chemical profiling of three parts (leaf, stem, and root) of E. chrysantha was performed using UPLC-Q-TOF-MS/MS spectrometry, followed by metabolomics analysis. Based on the chemical profiles and glucose uptake activity, compounds were isolated from the root. Their structures were elucidated using spectroscopic techniques, including UV, NMR, and mass spectrometry. The glucose uptake activity of the isolated compounds was assessed using a 2-NBDG assay.
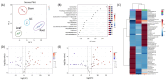
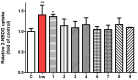
Results: Metabolic analysis revealed distinct chemical compositions among the plant parts. Dimeric coumarins and biflavonoids were abundant in the root, whereas flavonoid monomers were predominant in the leaf. Bioactivity-guided isolation yielded nine compounds (1-9), among which compound 1, a newly identified coumarin glycoside, exhibited significant glucose uptake-enhancing activity. Molecular docking analysis further suggested that compound 1 activates AMPK through an allosteric site, thereby promoting glucose uptake.
Conclusions: These findings provide a comprehensive chemical and metabolomic characterization of E. chrysantha and highlight its potential as a functional food ingredient for glucose-lowering effects.
Keywords: Edgeworthia chrysantha; Thymelaeaceae; anti-diabetic; diabetes; glucose uptake; metabolomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Phytochemical Screening and Biological Activities of Lippia multiflora Moldenke.Molecules. 2025 Jul 7;30(13):2882. doi: 10.3390/molecules30132882. Molecules. 2025. PMID: 40649396 Free PMC article.
-
Metabolomics Analysis on Different Parts of Ligustrum lucidum Ait. Based on UPLC-Q-TOF-MS.J AOAC Int. 2025 Sep 1;108(5):786-795. doi: 10.1093/jaoacint/qsaf057. J AOAC Int. 2025. PMID: 40489654
-
Chemometrics-driven LC-MS analysis reveals key metabolites and chemical diversity in Abrus cantoniensis Hance stems and leaves.J Chromatogr A. 2025 Sep 27;1759:466233. doi: 10.1016/j.chroma.2025.466233. Epub 2025 Jul 18. J Chromatogr A. 2025. PMID: 40749535
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Integrating LC-MS/MS and Molecular Networking for Advance Analysis and Comprehensive Flavonoids Annotation.J Sep Sci. 2025 Jul;48(7):e70230. doi: 10.1002/jssc.70230. J Sep Sci. 2025. PMID: 40678874 Review.
References
MeSH terms
Substances
Grants and funding
- RS-2023-00218616/Basic Science Research Program Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in Korea
- RS-2022-NR070150/Basic Science Research Program Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in Korea
LinkOut - more resources
Full Text Sources

