Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation
- PMID: 40872210
- PMCID: PMC12389575
- DOI: 10.3390/plants14162586
Optimizing Growth Regulator Concentrations for Cannabis sativa L. Micropropagation
Abstract
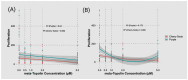
In this study, the effect of growth regulators on shoot proliferation and rooting were evaluated to develop an efficient micropropagation protocol for the Cannabis sativa L. cultivars 'Cherry Soda' and 'Purple'. Apical meristems were isolated from actively growing shoots of stock plants and transferred to Driver and Kuniyuki Walnut (DKW) culture medium containing either 0.0, 0.5, 1.0, 2.0, or 5.0 μM meta-Topolin to study their shoot proliferation response. Resulting shoot cultures were transferred to medium containing varying levels of Indole Acetic Acid (IAA), Indole Butyric Acid (IBA), or Naphthalene Acetic Acid (NAA), solely or in combination, and were subjected to a 10-day dark incubation followed by a 16 h/8 h light/dark period to identify the best treatment for root production. Among the different shoot proliferation treatments studied, the maximum number of shoots was produced on the control medium that was devoid of any meta-Topolin. Cultures grown on medium containing 5.0 μM meta-Topolin exhibited hyperhydricity, where shoots appeared translucent and pale green in color; were characterized by water-soaked lesions; and leaves appeared curled and brittle in contrast to healthy looking cultures. Among the various rooting treatments studied, shoots grown in the dark for 10 days exhibited the highest frequency of rooting on medium containing 4.0 μM NAA or 6.0 μM IBA + 1.0 μM NAA. Full developed plants with a robust shoot and root system were transferred to soil, acclimatized under conditions for high humidity, and then transferred to ambient conditions in 4 weeks. The micropropagation protocol developed here allows for rapid multiplication of disease-free plants in C. sativa cultivars.
Keywords: auxin; contamination; cytokinin; hyperhydricity; industrial hemp.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Small E., Cronquist A. A Practical and Natural Taxonomy for Cannabis. Taxon. 1976;25:405–435. doi: 10.2307/1220524. - DOI
-
- Clarke R.C., Merlin M.D. Cannabis: Evolution and Ethnobotany. Plant Ecol. Evol. 2013;147:149.
-
- Clarke R.C., Merlin M.D. Cannabis Domestication, Breeding History, Present-Day Genetic Diversity, and Future Prospects. Crit. Rev. Plant Sci. 2016;35:293–327. doi: 10.1080/07352689.2016.1267498. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

