Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry
- PMID: 40872275
- PMCID: PMC12389261
- DOI: 10.3390/pathogens14080765
Influence of Sex and 1,25α Dihydroxyvitamin D3 on SARS-CoV-2 Infection and Viral Entry
Abstract
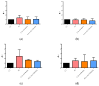
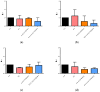
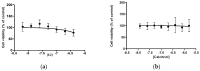
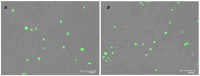
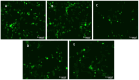
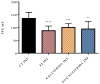
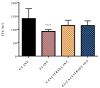
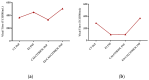
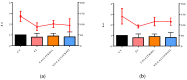
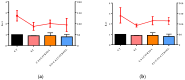
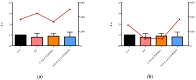
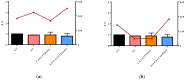
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is the etiologic agent that causes the coronavirus disease (COVID-19) identified in Wuhan, in 2019. Men are more prone to developing severe manifestations than women, suggesting a possible crucial role of sex hormones. 17,β-Estradiol (E2) and 1,25 α dihydroxyvitamin D3 (calcitriol) act upon gene pathways as immunomodulators in several infectious respiratory diseases. In this study, we aimed to evaluate the influence of E2 and calcitriol on the VSV-based pseudovirus SARS-CoV-2 and SARS-CoV-2 infection in vitro. We infected Vero E6 cells with the recombinant VSV-based pseudovirus SARS-CoV-2 and the SARS-CoV-2 viruses according to the pre-treatment and pre-post-treatment models. The Angiotensin-Converting Enzyme 2 (ACE2) and Vitamin D Receptor (VDR) gene expression did not change under different treatments. The VSV-based pseudovirus SARS-CoV-2 infection showed a significant decrease in the focus-forming unit count in the presence of E2 and calcitriol (either alone or in combination) in the pre-treatment model, while in the pre-post-treatment model, the infection was inhibited only in the presence of E2. Th SARS-CoV-2 infection highlighted a decrease in viral titres in the presence of E2 and calcitriol only in the pre-post-treatment model. 17,β-Estradiol and calcitriol can exert an inhibitory effect on SARS-CoV-2 infections, demonstrating their protective role against viral infections.
Keywords: 1,25α dihydroxyvitamin D3; COVID-19; E2; SARS-CoV-2; calcitriol; estrogen; sex; sex hormones; viral entry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

