Antiviral Activity of Medicinal Plant Extracts Vitex negundo and Macaranga tanarius Against SARS-CoV-2
- PMID: 40872330
- PMCID: PMC12388886
- DOI: 10.3390/pathogens14080820
Antiviral Activity of Medicinal Plant Extracts Vitex negundo and Macaranga tanarius Against SARS-CoV-2
Abstract
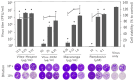
Natural products possess a wide range of biological and biochemical potentials, with plant-derived compounds being significant sources for discovering new drugs. In this study, extracts of Vitex negundo and Macaranga tanarius prepared with different solvents were tested for their antiviral activity against the original SARS-CoV-2 Wuhan strain and its variants using plaque assay, quantitative real time RT-PCR, and immunofluorescence assay (IFA). Our results showed that at their maximum non-toxic concentrations, Vitex-Dichloromethane (DCM) and Macaranga extracts significantly inhibited SARS-CoV-2 Wuhan strain growth in Vero E6 cells, showing a 5-log reduction in plaque assay and confirmed by IFA. Meanwhile, Vitex-Hexane showed moderate activity with a 2-log decrease. The inhibition was shown in a dose-dependent manner. The antiviral efficacy of these extracts was further demonstrated against various SARS-CoV-2 variants including Alpha, Beta, Delta, and Omicron. Both Vitex-DCM and Macaranga showed strong virucidal activity. In addition, Vitex-DCM and Macaranga inhibited the transcriptional activity of purified SARS-CoV-2 RdRp, indicating that RdRp inhibition may contribute to viral suppression as shown at the post-infection stage. Furthermore, combining Vitex-DCM or Macaranga with remdesivir showed a synergistic effect against SARS-CoV-2. These results suggest that Vitex negundo and Macaranga tanarius extracts are promising candidates for anti-SARS-CoV-2 treatments. Their synergy with remdesivir also underscores the potential of drug combinations in fighting SARS-CoV-2 and preventing the emergence of mutant variants.
Keywords: Macaranga tanarius; SARS-CoV-2; Vitex negundo; antiviral.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

