Auricularia auricula's Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin-1-Mediated Immunomodulation and Microbiota Remodeling
- PMID: 40872478
- PMCID: PMC12389670
- DOI: 10.3390/ph18081085
Auricularia auricula's Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin-1-Mediated Immunomodulation and Microbiota Remodeling
Abstract
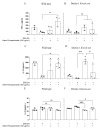
Background/Objectives: Ulcerative colitis (UC) is characterized by the interplay between immune responses and dysbiosis in disease development. Aiming to provide additional insights into disease development and potential treatment strategies, the present study investigates the local effect of oral treatment with polysaccharides obtained from Auricularia auricula's submerged culture in an experimental model of DSS-induced colitis and its impact on lesion resolution. Methods: The structure and monosaccharide composition of Auricularia polysaccharides were characterized through Nuclear Magnetic Resonance (NMR). To evaluate the effect of this polysaccharide on the murine model, wild-type and Dectin-1 knockout mice were treated or not with the exopolysaccharide (EPS) while under DSS consumption. During the experimental period, feces samples were collected to evaluate microbial shifts during disease development, and, finally, the colonic tissue was analyzed to assess the inflammatory process and cytokine production. Results: The EPS composition showed a polymeric mixture of glucans and fucogalactomannans. The treatment of the wild-type DSS-induced colitis group improved the inflammatory response by increasing gut-homeostatic cytokines, such as interleukin-10 (IL-10) and tumor necrosis factor-alpha (TNF-α). The Dectin-1 KO mice group did not show the same enhancement after EPS treatment. The microbiome analysis revealed a difference in the genotype, and the treatment modified the DSS microbiome modulation, with nine and four ASVs in WT and Dectin-1 KO mice, respectively. Conclusions: The EPS treatment demonstrated therapeutic potential in treating inflammatory intestinal diseases by modulating cytokine secretion and microbiota composition, which is dependent on the Dectin-1 receptor's carbohydrate recognition.
Keywords: microbiome; mushroom polysaccharide; prebiotics; ulcerative colitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Grants and funding
- 130898/2020-1 and 305584/2023-5/National Council for Scientific and Technological Development
- 193.00000029/2019-53 and 00193.00002346/2022-18/Fundação de Apoio Pesquisa do Distrito Federal
- 405934/2022-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Coordenação de Aperfeiçoamento do Pessoal do Ensino Superior (CAPES) (The National Institute of Science and Technology - INCT Funvir)
LinkOut - more resources
Full Text Sources
Research Materials

