Phytocannabinoids as Novel SGLT2 Modulators for Renal Glucose Reabsorption in Type 2 Diabetes Management
- PMID: 40872492
- PMCID: PMC12389431
- DOI: 10.3390/ph18081101
Phytocannabinoids as Novel SGLT2 Modulators for Renal Glucose Reabsorption in Type 2 Diabetes Management
Abstract
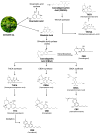
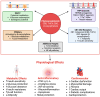
Background: Sodium-glucose cotransporter 2 (SGLT2) inhibitors have transformed type 2 diabetes mellitus (T2DM) management by promoting glucosuria, lowering glycated hemoglobin (HbA1c), blood pressure, and weight; however, their use is limited by genitourinary infections and ketoacidosis. Phytocannabinoids-bioactive compounds from Cannabis sativa-exhibit multi-target pharmacology, including interactions with cannabinoid receptors, Peroxisome Proliferator-Activated Receptors (PPARs), Transient Receptor Potential (TRP) channels, and potentially SGLT2. Objective: To evaluate the potential of phytocannabinoids as novel modulators of renal glucose reabsorption via SGLT2 and to compare their efficacy, safety, and pharmacological profiles with synthetic SGLT2 inhibitors. Methods: We performed a narrative review encompassing the following: (1) the molecular and physiological roles of SGLT2; (2) chemical classification, natural sources, and pharmacokinetics/pharmacodynamics of major phytocannabinoids (Δ9-Tetrahydrocannabinol or Δ9-THC, Cannabidiol or CBD, Cannabigerol or CBG, Cannabichromene or CBC, Tetrahydrocannabivarin or THCV, and β-caryophyllene); (3) in silico docking and drug-likeness assessments; (4) in vitro assays of receptor binding, TRP channel modulation, and glucose transport; (5) in vivo rodent models evaluating glycemic control, weight change, and organ protection; (6) pilot clinical studies of THCV and case reports of CBD/BCP; (7) comparative analysis with established synthetic inhibitors. Results: In silico studies identify high-affinity binding of several phytocannabinoids within the SGLT2 substrate pocket. In vitro, CBG and THCV modulate SGLT2-related pathways indirectly via TRP channels and CB receptors; direct IC50 values for SGLT2 remain to be determined. In vivo, THCV and CBD demonstrate glucose-lowering, insulin-sensitizing, weight-reducing, anti-inflammatory, and organ-protective effects. Pilot clinical data (n = 62) show that THCV decreases fasting glucose, enhances β-cell function, and lacks psychoactive side effects. Compared to synthetic inhibitors, phytocannabinoids offer pleiotropic benefits but face challenges of low oral bioavailability, polypharmacology, inter-individual variability, and limited large-scale trials. Discussion: While preclinical and early clinical data highlight phytocannabinoids' potential in SGLT2 modulation and broader metabolic improvement, their translation is impeded by significant challenges. These include low oral bioavailability, inconsistent pharmacokinetic profiles, and the absence of standardized formulations, necessitating advanced delivery system development. Furthermore, the inherent polypharmacology of these compounds, while beneficial, demands comprehensive safety assessments for potential off-target effects and drug interactions. The scarcity of large-scale, well-controlled clinical trials and the need for clear regulatory frameworks remain critical hurdles. Addressing these aspects is paramount to fully realize the therapeutic utility of phytocannabinoids as a comprehensive approach to T2DM management. Conclusion: Phytocannabinoids represent promising multi-target agents for T2DM through potential SGLT2 modulation and complementary metabolic effects. Future work should focus on pharmacokinetic optimization, precise quantification of SGLT2 inhibition, and robust clinical trials to establish efficacy and safety profiles relative to synthetic inhibitors.
Keywords: SGLT2; cannabidiol; in silico docking; phytocannabinoids; renal glucose reabsorption; tetrahydrocannabivarin; type 2 diabetes mellitus; β-caryophyllene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- D’Andrea E., Wexler D.J., Kim S.C., Paik J.M., Alt E., Patorno E. Comparing Effectiveness and Safety of SGLT2 Inhibitors vs DPP-4 Inhibitors in Patients with Type 2 Diabetes and Varying Baseline HbA1c Levels. JAMA Intern. Med. 2023;183:242–254. doi: 10.1001/jamainternmed.2022.6664. - DOI - PMC - PubMed
-
- Kanbay M., Tapoi L., Ureche C., Tanriover C., Cevik E., Demiray A., Afsar B., Cherney D.Z.I., Covic A. Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Hemoglobin and Hematocrit Levels in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. Urol. Nephrol. 2022;54:827–841. doi: 10.1007/s11255-021-02943-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

