Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain
- PMID: 40872550
- PMCID: PMC12389141
- DOI: 10.3390/ph18081159
Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain
Abstract
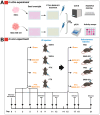
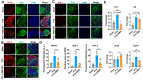
Background/Objectives: Chemotherapy-induced neuropathic pain (CINP) represents a critical challenge in oncology, emerging as a common and debilitating side effect of widely used chemotherapeutic agents, such as paclitaxel (PTX). Current therapeutic interventions and preventive strategies for CINP are largely insufficient, as they fail to address the underlying peripheral nerve damage, highlighting an urgent need for the development of new drugs. This study aimed to investigate the dual-function effects on normal cell protection and tumor suppression of BMX-001, a redox-active manganese metalloporphyrin that has demonstrated antioxidant and anti-inflammatory properties, which offers potential in protecting central nervous system tissues and treating CINP. Methods: This study assessed BMX-001's different roles in protecting normal cells while acting as a pro-oxidant and pro-inflammatory molecule in cancer cells in vitro. We also evaluated its neuroprotective effect in preclinical PTX-induced CINP models in vivo. Results: Our results showed significant reductions in mechanical and cold allodynia, decreased pro-inflammatory cytokine levels, and restored antioxidant capacity in peripheral nerves and dorsal root ganglia (DRGs) following BMX-001 treatment. Conclusions: Overall, our study highlights the therapeutic potential of BMX-001 to mitigate CINP and enhance anticancer efficiency. Its dual-selective mechanism supports the future clinical investigation of BMX-001 as a novel adjunct to chemotherapeutic regimens.
Keywords: BMX-001; neuropathy; paclitaxel; reactive oxygen species; satellite glial cells.
Conflict of interest statement
Oberley-Deegan is a consultant for BioMimetix.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Desloratadine ameliorates paclitaxel-induced peripheral neuropathy and hypersensitivity reactions in mice.Acta Pharmacol Sin. 2024 Oct;45(10):2061-2076. doi: 10.1038/s41401-024-01301-z. Epub 2024 May 24. Acta Pharmacol Sin. 2024. PMID: 38789495
-
TLR9 in satellite glial cells promotes paclitaxel-induced neuropathic pain by reducing Kir4.1 transcription through histone methylation activation.Brain Behav Immun. 2025 Aug;128:65-82. doi: 10.1016/j.bbi.2025.03.037. Epub 2025 Mar 31. Brain Behav Immun. 2025. PMID: 40174869
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Hiramoto S., Asano H., Miyamoto T., Takegami M., Kawabata A. Risk factors and pharmacotherapy for chemotherapy-induced peripheral neuropathy in paclitaxel-treated female cancer survivors: A retrospective study in Japan. PLoS ONE. 2021;16:e0261473. doi: 10.1371/journal.pone.0261473. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

