In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines
- PMID: 40872568
- PMCID: PMC12388873
- DOI: 10.3390/ph18081177
In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines
Abstract
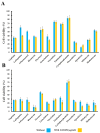
Background/Objectives: Annona muricata (AM), commonly known as soursop or guanabana, has long been used in traditional medicine for its purported anticancer properties. However, scientific studies evaluating its potential enhancing or additive effects with conventional antineoplastic drugs (ADs) remain limited. This study aimed to assess the cytotoxic effects of an aqueous AM infusion alone and in combination with standard ADs in cancer cell lines, while also evaluating its safety in healthy cells. Additionally, we explored the potential molecular interactions of AM metabolites with therapeutic targets using silico modeling. Methods: An AM infusion (125 and 250 µg/mL) was tested on two cancer cell lines-MDA-MB-231 (human triple-negative breast cancer) and TC-1 (murine HPV16-positive cancer)-as well as healthy human leukocytes and a non-tumorigenic mouse lung cell line. Cell viability was assessed using the Alamar Blue™ assay. The combined effects of AM with multiple first-line ADs were evaluated. In silico molecular docking was performed with Molegro Virtual Docker to assess the interaction of AM metabolites (quercetin and hyperoside) with the A2B adenosine receptor. Additionally, the physicochemical properties of 13 AD were analyzed to explore correlations with cytotoxic outcomes. Results: AM infusion alone exhibited low cytotoxicity in both cancer and healthy cell types. However, when combined with ADs, it enhanced cytotoxic effects in cancer cells while sparing healthy cells at the evaluated concentrations. Docking studies revealed strong interactions between quercetin and hyperoside (major metabolites in the AM infusion) and the A2B receptor, supporting a possible mechanistic explanation for the observed effects. Conclusions: AM infusion may act as a chemical modulator, potentiating the effects of conventional ADs in cancer cells while preserving normal cell viability. These findings encourage further preclinical exploration of AM as a complementary agent in integrative oncology.
Keywords: adjuvant; antineoplastic drug; breast cancer; folk medicine; guanabana; infusion; traditional medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zubaidi S.N., Nani H.M., Kamal M.S.A., Qayyum T.A., Maarof S., Afzan A., Misnan N.M., Hamezah H.S., Baharum S.N., Mediani A. Annona muricata: Comprehensive Review on the Ethnomedicinal, Phytochemistry, and Pharmacological Aspects Focusing on Antidiabetic Properties. Life. 2023;13:353. doi: 10.3390/life13020353. - DOI - PMC - PubMed
-
- Hernandez-Fuentes G.A., Delgado-Enciso O.G., Larios-Cedeño E.G., Sánchez-Galindo J.M., Ceballos-Magaña S.G., Pineda-Urbina K., Alcalá-Pérez M.A., Magaña-Vergara N.E., Delgado-Enciso J., Díaz-Llerenas U., et al. Comparative Analysis of Infusions and Ethanolic Extracts of Annona muricata Leaves from Colima, Mexico: Phytochemical Profile and Antioxidant Activity. Life. 2024;14:1702. doi: 10.3390/life14121702. - DOI - PMC - PubMed
-
- Fuentes L.M.H., González E.M., Magaña M.d.L.G., Esparza L.M.A., González Y.N., Villagrán Z., Torres S.G., Monreal J.J.V., Flores D.A.M. Current Situation and Perspectives of Fruit Annonaceae in Mexico: Biological and Agronomic Importance and Bioactive Properties. Plants. 2021;11:7. doi: 10.3390/plants11010007. - DOI - PMC - PubMed
-
- Riley-Saldaña C.A., Cruz-Ortega M.D.R., Vázquez M.M., De-La-Cruz-Chacón I., Castro-Moreno M., González-Esquinca A.R. Acetogenins and Alkaloids during the Initial Development of Annona muricata L. (Annonaceae) Z. Fur Naturforschung Sect. C J. Biosci. 2017;72:497–506. doi: 10.1515/znc-2017-0060. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

