Targeting Trypanothione Synthetase and Trypanothione Reductase: Development of Common Inhibitors to Tackle Trypanosomatid Disease
- PMID: 40872574
- PMCID: PMC12389602
- DOI: 10.3390/ph18081182
Targeting Trypanothione Synthetase and Trypanothione Reductase: Development of Common Inhibitors to Tackle Trypanosomatid Disease
Abstract
Neglected Tropical Diseases (NTDs) encompass a range of disorders, including infectious diseases caused by viruses, bacteria, parasites, fungi, and toxins, mainly affecting underprivileged individuals in developing countries. Among the NTDs, those caused by parasites belonging to the Trypanosomatidae family are particularly impacting and require attention, since the lack of financial incentives has led to constraints on the development of novel drugs to tackle them effectively. To circumvent the minor advances in drug discovery in this area, academic research emerges as a crucial player, namely through the identification and validation of new drug targets, thereby contributing to the development of more efficient, safe, and less expensive therapies against Trypanosomatidae infections. Noteworthy, this is a matter of utmost urgency since these diseases are endemic in countries with low socioeconomic standards. This review provides a comprehensive understanding of the current paradigm of NTDs caused by parasites belonging to the Trypanosomatidae family, addressing the ongoing limitations and challenges associated to the current chemotherapy solutions for these diseases and discussing the opportunities unravelled by recent research that led to the identification of new biomolecular targets that are common to Trypanosomatidae parasites. Among these, the unique properties of Trypanothione Synthetase (TryS) and Trypanothione Reductase (TryR), two key protozoan enzymes that are essential for the survival of Trypanosoma and Leishmania parasites, will be emphasised. In addition to a critical analysis of the latest advances in the discovery of novel molecules capable of inhibiting TryS and TryR, the possibility of dual targeting through a combination of TryS and TryR inhibitors will be addressed.
Keywords: inhibitors; leishmaniases; neglected tropical diseases; trypanosomiasis; trypanothione reductase; trypanothione synthetase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









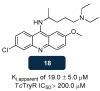





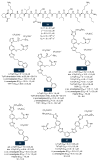



References
-
- Forbes K., Basáñez M., Hollingsworth T., Anderson R. Introduction to the special issue: Challenges and opportunities in the fight against neglected tropical diseases: A decade from the London Declaration on NTDs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023;378:20220272. doi: 10.1098/rstb.2022.0272. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

