Exercise-Induced Muscle-Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging
- PMID: 40872612
- PMCID: PMC12389176
- DOI: 10.3390/ph18081222
Exercise-Induced Muscle-Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging
Abstract
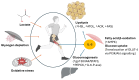
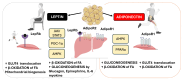
Regular physical activity induces a dynamic crosstalk between skeletal muscle and adipose tissue, modulating the key molecular pathways that underlie metabolic flexibility, mitochondrial function, and inflammation. This review highlights the role of myokines and adipokines-particularly IL-6, irisin, leptin, and adiponectin-in orchestrating muscle-adipose tissue communication during exercise. Exercise stimulates AMPK, PGC-1α, and SIRT1 signaling, promoting mitochondrial biogenesis, fatty acid oxidation, and autophagy, while also regulating muscle hypertrophy through the PI3K/Akt/mTOR and Wnt/β-catenin pathways. Simultaneously, adipose-derived factors like leptin and adiponectin modulate skeletal muscle metabolism via JAK/STAT3 and AdipoR1-mediated AMPK activation. Additionally, emerging exercise mimetics such as the mitochondrial-derived peptide MOTS-c and myostatin inhibitors are highlighted for their roles in increasing muscle mass, the browning of white adipose tissue, and improving systemic metabolic function. The review also addresses the role of anti-inflammatory compounds, including omega-3 polyunsaturated fatty acids and low-dose aspirin, in mitigating NF-κB and IL-6 signaling to protect mitochondrial health. The resulting metabolic flexibility, defined as the ability to efficiently switch between lipid and glucose oxidation, is enhanced through repeated exercise, counteracting age- and disease-related mitochondrial and functional decline. Together, these adaptations demonstrate the importance of inter-tissue signaling in maintaining energy homeostasis and preventing sarcopenia, obesity, and insulin resistance. Finally, here we propose a stratified treatment algorithm based on common age-related comorbidities, offering a framework for precision-based interventions that may offer a promising strategy to preserve metabolic plasticity and delay the age-associated decline in cardiometabolic health.
Keywords: IL-6; adipokines; adiponectin; irisin; leptin; metabolic flexibility; mitochondrial biogenesis; myokines; skeletal muscle–adipose tissue crosstalk.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Impact of Adipose Tissue and Lipids on Skeletal Muscle in Sarcopenia.J Cachexia Sarcopenia Muscle. 2025;16(4):e70000. doi: 10.1002/jcsm.70000. J Cachexia Sarcopenia Muscle. 2025. PMID: 40641114 Free PMC article. Review.
-
Modulating Benign Prostatic Hyperplasia Through Physical Activity-The Emerging Role of Myokines: A Narrative Review.Medicina (Kaunas). 2025 Jul 28;61(8):1362. doi: 10.3390/medicina61081362. Medicina (Kaunas). 2025. PMID: 40870407 Free PMC article. Review.
-
Leptin mediates the regulation of muscle mass and strength by adipose tissue.J Physiol. 2022 Aug;600(16):3795-3817. doi: 10.1113/JP283034. Epub 2022 Aug 2. J Physiol. 2022. PMID: 35844058 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
References
-
- Joseph A., Adhihetty P.J., Buford T.W., Wohlgemuth S.E., Lees H.A., Nguyen L.M.-D., Aranda J.M., Sandesara B.D., Pahor M., Manini T.M., et al. The Impact of Aging on Mitochondrial Function and Biogenesis Pathways in Skeletal Muscle of Sedentary High- and Low-functioning Elderly Individuals. Aging Cell. 2012;11:801–809. doi: 10.1111/j.1474-9726.2012.00844.x. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

