Equine Pituitary Pars Intermedia Dysfunction
- PMID: 40872730
- PMCID: PMC12390634
- DOI: 10.3390/vetsci12080780
Equine Pituitary Pars Intermedia Dysfunction
Abstract
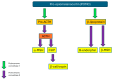
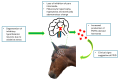
Pituitary pars intermedia dysfunction (PPID) is a common, slowly progressive, neurodegenerative disorder of the older horse. Oxidative damage to the hypothalamic periventricular neurons results in loss of dopaminergic inhibition of the pars intermedia region of the pituitary gland. Consequently, there is increased production of the pro-opiomelanocortin (POMC)-derived hormones normally produced by this region, as well as initial melanocyte hypertrophy and hyperplasia, followed by adenomatous change. Clinical signs that are highly suggestive of the disease are generalised and regional hypertrichosis and delayed/abnormal coat shedding. Numerous clinical signs provide a moderate clinical suspicion, including hyperhidrosis, abnormal fat distribution/regional adiposity, epaxial muscle atrophy/loss of topline, laminitis, weight loss, recurrent infections, behavioural changes/lethargy, polyuria and polydipsia, a pot-bellied appearance, bulging supraorbital fat pads, reduced wound healing, lordosis and infertility. In all animals, a diagnosis of PPID is made based on the signalment, clinical signs and results of further diagnostic tests, with age being a crucial factor to consider. Currently recommended further diagnostic tests are measurement of basal adrenocorticotrophic hormone (ACTH) concentrations (all year) and evaluation of the ACTH response to thyrotrophin-releasing hormone (TRH) using seasonally adjusted references intervals (non-autumn). Animals should also be tested for insulin dysregulation, as laminitis risk in PPID is associated with hyperinsulinaemia. PPID can be managed but not cured; it is a lifelong condition. The individual clinical signs can be managed, e.g., clipping the excessive haircoat and providing unrestricted access to water for individuals with polydipsia. Alternatively, pharmacological management can be employed, and the dopamine-2 receptor agonist pergolide is licensed/approved for the treatment of equine PPID. This should be prescribed in combination with dietary recommendations based on the body condition score and insulin sensitivity status of the individual animal.
Keywords: ACTH; dysfunction; insulin; pars intermedia; pergolide; pituitary.
Conflict of interest statement
The author declares no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

