Tracking the Spatial and Functional Dispersion of Vaccine-Related Canine Distemper Virus Genotypes: Insights from a Global Scoping Review
- PMID: 40872760
- PMCID: PMC12390544
- DOI: 10.3390/v17081045
Tracking the Spatial and Functional Dispersion of Vaccine-Related Canine Distemper Virus Genotypes: Insights from a Global Scoping Review
Abstract
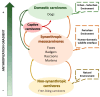
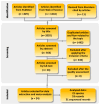
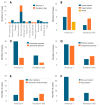
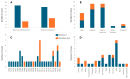
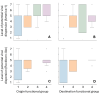
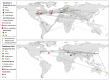
Canine morbillivirus (CDV), the cause of canine distemper, is a pathogen affecting many hosts. While modified live virus (MLV) vaccines are crucial for controlling the disease in dogs, cases of vaccine-related infections have been found in both domestic and wild animals. Specifically, the America-1 and Rockborn-like vaccine genotypes are concerning due to their spread and ability to transmit between different species. This study conducted a review and analysis of molecular detections of these strains in various carnivores (domestic, captive, synanthropic, and wild species). This study used a conceptual model considering host ecology and the domestic-wild interface to evaluate plausible transmission connections over time using Linear Directional Mean (LDM) and Weighted Mean Centre (WMC) methods. Statistical analyses examined the relationship between how likely a strain is to spread and factors like host type and vaccination status. The findings showed that the America-1 genotype spread in a more organised way, with domestic dogs being the main source and recipient, bridging different environments. Synanthropic mesocarnivores also played this same role, with less intensity. America-1 was most concentrated in the North Atlantic and Western Europe. In contrast, the Rockborn-like strain showed a more unpredictable and restricted spread, residual circulation from past use rather than ongoing spread. Species involved in vaccine-related infections often share characteristics like generalist behaviour, social living, and a preference for areas where domestic animals and wildlife interact. We did not find a general link between a host vaccination status and the likelihood of the strain spreading. The study emphasised the ongoing risk of vaccine-derived strains moving from domestic and synanthropic animals to vulnerable wild species, supporting the need for improved vaccination approaches. Mapping these plausible transmission routes can serve as a basis for targeted surveillance, not only of vaccine-derived strains, but of any other circulating genotype.
Keywords: America-1; CDV; Morbillivirus canis; Rockborn-like; multi-host pathogen; spillover; spread; surveillance; transmission; vaccine genotypes; vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Canine Distemper Virus in Mexico: A Risk Factor for Wildlife.Viruses. 2025 Jun 3;17(6):813. doi: 10.3390/v17060813. Viruses. 2025. PMID: 40573404 Free PMC article. Review.
-
Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild-Domestic Canid Interface in Aburrá Valley, Colombia.Viruses. 2025 Apr 29;17(5):649. doi: 10.3390/v17050649. Viruses. 2025. PMID: 40431661 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Are Domestic Dogs (Canis familiaris) the Family Scapegoats? A Systematic Review of Canine Distemper Virus in African Wildlife, 1978-2021.J Wildl Dis. 2025 Jan 1;61(1):1-16. doi: 10.7589/JWD-D-24-00017. J Wildl Dis. 2025. PMID: 39603253
-
Recombinant canine distemper virus expressing virus-like particle VP2 protein of mink enteritis virus protects minks against lethal challenges of both viruses.Vet Microbiol. 2025 Aug;307:110625. doi: 10.1016/j.vetmic.2025.110625. Epub 2025 Jun 26. Vet Microbiol. 2025. PMID: 40609500
References
-
- International Committee on Taxonomy of Viruses (ICTV) [(accessed on 10 May 2024)]. Available online: https://ictv.global/taxonomy.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

