The Evolution of Cell Culture Systems to Study Hepatitis B Virus Pathogenesis and Antiviral Susceptibility
- PMID: 40872772
- PMCID: PMC12390701
- DOI: 10.3390/v17081057
The Evolution of Cell Culture Systems to Study Hepatitis B Virus Pathogenesis and Antiviral Susceptibility
Abstract
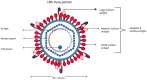
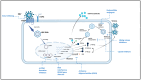
The global burden of hepatitis B virus (HBV) remains high, with ongoing concerted efforts to eliminate viral hepatitis as a public health concern by 2030. The absence of curative treatment against HBV makes it an active area of research to further study HBV pathogenesis. In vitro cell culture systems are essential in exploration of molecular mechanisms for HBV propagation and the development of therapeutic targets for antiviral agents. The lack of an efficient cell culture system is one of the challenges limiting the development and study of novel antiviral strategies for HBV infection. However, the evolution of cell culture systems to study HBV pathogenesis and treatment susceptibility in vitro has made a significant contribution to public health. The currently available cell culture systems to grow HBV have their advantages and limitations, requiring further optimization. The discovery of sodium taurocholate co-transporting polypeptide (NTCP) as a receptor for HBV was a major breakthrough for the development of a robust cell model, allowing the study of de novo HBV infection through NTCP expression in the HepG2 hepatoma cell line. This review is aimed at highlighting the evolution of cell culture systems to study HBV pathogenesis and in vitro treatment susceptibility.
Keywords: HepG2-NTCP cells; cell culture systems; hepatitis B virus (HBV).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The loss of hepatitis B virus receptor NTCP/SLC10A1 in human liver cancer cells is due to epigenetic silencing.J Virol. 2024 Oct 22;98(10):e0118724. doi: 10.1128/jvi.01187-24. Epub 2024 Sep 19. J Virol. 2024. PMID: 39297647 Free PMC article.
-
Molecular mechanisms of hepatitis B virus entry inhibition by a bile acid derivative INT-767 binding to the preS1 region.Antiviral Res. 2025 Aug;240:106213. doi: 10.1016/j.antiviral.2025.106213. Epub 2025 Jun 11. Antiviral Res. 2025. PMID: 40513783
-
RXR agonist S169 inhibits HBV/HDV entry in vitro by disrupting KIF4-dependent NTCP trafficking.Antiviral Res. 2025 Aug;240:106214. doi: 10.1016/j.antiviral.2025.106214. Epub 2025 Jun 14. Antiviral Res. 2025. PMID: 40523562
-
Therapeutic interventions aimed at cccDNA: unveiling mechanisms and evaluating the potency of natural products.Front Cell Infect Microbiol. 2025 Jun 17;15:1598872. doi: 10.3389/fcimb.2025.1598872. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40599653 Free PMC article. Review.
-
Pharmacological interventions for acute hepatitis B infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 21;3(3):CD011645. doi: 10.1002/14651858.CD011645.pub2. Cochrane Database Syst Rev. 2017. PMID: 28321877 Free PMC article.
References
-
- WHO Hepatitis B. [(accessed on 2 July 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- CDC Fast Facts: Global Hepatitis B Vaccination. [(accessed on 2 July 2024)]; Available online: https://www.cdc.gov/global-hepatitis-b-vaccination/data-research/index.h....
-
- Faniyi A.A., Okesanya O.J., Manirambona E., Oso T.A., Olaleke N.O., Nukpezah R.N., Ilesanmi A.O., Lucero-Prisno D.E., III Advancing public health policies to combat Hepatitis B in Africa: Challenges, advances, and recommendations for meeting 2030 targets. J. Med. Surg. Public Health. 2024;2:100058. doi: 10.1016/j.glmedi.2024.100058. - DOI
-
- Seremba E., Ssempijja V., Kalibbala S., Gray R.H., Wawer M.J., Nalugoda F., Casper C., Phipps W., Ocama P., Serwadda D. Hepatitis B incidence and prevention with antiretroviral therapy among HIV-positive individuals in Uganda. Aids. 2017;31:781–786. doi: 10.1097/QAD.0000000000001399. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

