Development of COVID-19 Vaccine Candidates Using Attenuated Recombinant Vesicular Stomatitis Virus Vectors with M Protein Mutations
- PMID: 40872776
- PMCID: PMC12390721
- DOI: 10.3390/v17081062
Development of COVID-19 Vaccine Candidates Using Attenuated Recombinant Vesicular Stomatitis Virus Vectors with M Protein Mutations
Abstract
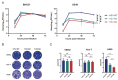
Recombinant vesicular stomatitis virus (rVSV) is a promising viral vaccine vector for addressing the COVID-19 pandemic. Inducing mucosal immunity via the intranasal route is an ideal strategy for rVSV-based vaccines, but it requires extremely stringent safety standards. In this study, we constructed two rVSV variants with amino acid mutations in their M protein: rVSV-M2 with M33A/M51R mutations and rVSV-M4 with M33A/M51R/V221F/S226R mutations, and developed COVID-19 vaccines based on these attenuated vectors. By comparing viral replication capacity, intranasal immunization, intracranial injection, and blood cell counts, we demonstrated that the M protein mutation variants exhibit significant attenuation effects both in vitro and in vivo. Moreover, preliminary investigations into the mechanisms of virus attenuation revealed that these attenuated viruses can induce a stronger type I interferon response while reducing inflammation compared to the wild-type rVSV. We developed three candidate vaccines against SARS-CoV-2 using the wildtype VSV backbone with either wild-type M (rVSV-JN.1) and two M mutant variants (rVSV-M2-JN.1 and rVSV-M4-JN.1). Our results confirmed that rVSV-M2-JN.1 and rVSV-M4-JN.1 retain strong immunogenicity while enhancing safety in hamsters. In summary, the rVSV variants with M protein mutations represent promising candidate vectors for mucosal vaccines and warrant further investigation.
Keywords: SARS-CoV-2; live attenuated vaccine; mucosal immunity; vesicular stomatitis virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kumar S.U., Priya N.M., Nithya S.R., Kannan P., Jain N., Kumar D.T., Magesh R., Younes S., Zayed H., Doss C.G.P. A review of novel coronavirus disease (COVID-19): Based on genomic structure, phylogeny, current shreds of evidence, candidate vaccines, and drug repurposing. 3 Biotech. 2021;11:198. doi: 10.1007/s13205-021-02749-0. - DOI - PMC - PubMed
-
- Fuchs J.D., Frank I., Elizaga M.L., Allen M., Frahm N., Kochar N., Li S., Edupuganti S., Kalams S.A., Tomaras G.D., et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090) Open Forum Infect. Dis. 2015;2:ofv082. doi: 10.1093/ofid/ofv082. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

