First Use of Phage Therapy in Canada for the Treatment of a Life-Threatening, Multidrug-Resistant Staphylococcus epidermidis Periprosthetic Joint Infection
- PMID: 40872831
- PMCID: PMC12390693
- DOI: 10.3390/v17081118
First Use of Phage Therapy in Canada for the Treatment of a Life-Threatening, Multidrug-Resistant Staphylococcus epidermidis Periprosthetic Joint Infection
Abstract
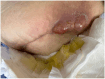
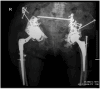
We describe the first use of phage therapy in Canada for the treatment of a life-threatening periprosthetic joint infection (PJI), with successful outcome. PJI is a devastating complication of joint replacement surgery, with high morbidity and mortality. Our patient presented with early sepsis from a chronic recalcitrant multidrug-resistant (MDR) Staphylococcus epidermidis hip PJI which had repeatedly failed standard therapy. She had previously undergone 10 operations of the right hip, and only three weeks after completing a prolonged course of daptomycin following her most recent hip revision, she developed a draining sinus tract. Given the high burden of disease, inability to achieve surgical source control, and lack of antibiotic treatment options for long-term suppressive therapy, bacteriophage (phage) therapy was pursued. The patient underwent irrigation and debridement with complex flap reconstruction: intraoperative tissue cultures again yielded MDR S. epidermidis. We developed a novel phage therapy protocol for this patient, with twice daily, intra-articular and intravenous (7 × 109 PFU/dose) phage delivery over a planned 14-day course. Complete healing of the wound with cessation of drainage occurred within one month after treatment. A marked improvement in right hip pain and mobility occurred within three months after treatment. Twelve months following phage treatment, there is normalization of serum inflammatory markers with diminished pain, increased mobility, and no recurrent surgery. Our patient continues to improve and is currently living independently at home, with sustained clinical control of infection.
Keywords: antibiotic resistance; periprosthetic joint infection; phage therapy.
Conflict of interest statement
M.A.A. is a consultant for BioFire USA and bioMérieux Canada. D.W.C. receives a salary award from the Faculty and Department of Medicine, University of Ottawa. G.A.S. is an unpaid advisor for Phiogen and has intellectual property licensed to Adaptive Phage Therapeutics and Precisio Biotix and contractual rights to receive royalties. B.W.M.C., M.T., M.J.P., T.A., S.L., H.A., N.F., K.L., T.J.L., Y.M.C., R.R., N.T., and S.S.T. are or were salaried employees at Cytophage Technologies Ltd.
Figures

References
-
- Pirnay J.P., Blasdel B.G., Bretaudeau L., Buckling A., Chanishvili N., Clark J.R., Corte-Real S., Debarbieux L., Dublanchet A., De Vos D., et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015;32:2173–2179. doi: 10.1007/s11095-014-1617-7. - DOI - PMC - PubMed
-
- Canadian Institute for Health Information. CJRR Annual Report: Hip and Knee Replacements in Canada, 2019–2020. [(accessed on 16 December 2024)]. Available online: https://www.cihi.ca/sites/default/files/document/cjrr-full-annual-report....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

