Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens
- PMID: 40872877
- PMCID: PMC12390594
- DOI: 10.3390/vaccines13080790
Bivalent Oral Vaccine Using Attenuated Salmonella Gallinarum Delivering HA and NA-M2e Confers Dual Protection Against H9N2 Avian Influenza and Fowl Typhoid in Chickens
Abstract
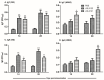
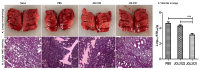
Background: Fowl typhoid (FT), a septicemic infection caused by Salmonella Gallinarum (SG), and H9N2 avian influenza are two economically important diseases that significantly affect the global poultry industry. Methods: We exploited the live attenuated Salmonella Gallinarum (SG) mutant JOL3062 (SG: ∆lon ∆pagL ∆asd) as a delivery system for H9N2 antigens to induce an immunoprotective response against both H9N2 and FT. To enhance immune protection against H9N2, a prokaryotic and eukaryotic dual expression plasmid, pJHL270, was employed. The hemagglutinin (HA) consensus sequence from South Korean avian influenza A virus (AIV) was cloned under the Ptrc promoter for prokaryotic expression, and the B cell epitope of neuraminidase (NA) linked with matrix protein 2 (M2e) was placed for eukaryotic expression. In vitro and in vivo expressions of the H9N2 antigens were validated by qRT-PCR and Western blot, respectively. Results: Oral immunization with JOL3121 induced a significant increase in SG and H9N2-specific serum IgY and cloacal swab IgA antibodies, confirming humoral and mucosal immune responses. Furthermore, FACS analysis showed increased CD4+ and CD8+ T cell populations. On day 28 post-immunization, there was a substantial rise in the hemagglutination inhibition titer in the immunized birds, demonstrating neutralization capabilities of immunization. Both IFN-γ and IL-4 demonstrated a significant increase, indicating a balance of Th1 and Th2 responses. Intranasal challenge with the H9N2 Y280 strain resulted in minimal to no clinical signs with significantly lower lung viral titer in the JOL3121 group. Upon SG wildtype challenge, the immunized birds in the JOL3121 group yielded 20% mortality, while 80% mortality was recorded in the PBS control group. Additionally, bacterial load in the spleen and liver was significantly lower in the immunized birds. Conclusions: The current vaccine model, designed with a host-specific pathogen, SG, delivers a robust immune boost that could enhance dual protection against FT and H9N2 infection, both being significant diseases in poultry, as well as ensure public health.
Keywords: H9N2; Salmonella; bivalent vaccine; dual expression system; fowl typhoid; immune response.
Conflict of interest statement
The authors confirm that there are no financial or personal conflicts that could have influenced the research presented in this manuscript.
Figures



References
-
- Aganja R.P., Bakhsh M., Senevirathne A., Kwon J., Lee J.H. Lipopolysaccharide structural modification in Salmonella Gallinarum targeting lipid a deacylation and O-antigen reduces virulence and endotoxicity with competitive protection against a wild-type challenge in chickens. Dev. Comp. Immunol. 2025;165:105343. doi: 10.1016/j.dci.2025.105343. - DOI - PubMed
-
- Moatasim Y., Kandeil A., Mostafa A., Elghaffar S.K.A., El Shesheny R., Elwahy A.H.M., Ali M.A. Single gene reassortment of highly pathogenic avian influenza A H5N1 in the low pathogenic H9N2 backbone and its impact on pathogenicity and infectivity of novel reassortant viruses. Arch. Virol. 2017;162:2959–2969. doi: 10.1007/s00705-017-3434-x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

