Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus
- PMID: 40872889
- PMCID: PMC12390407
- DOI: 10.3390/vaccines13080802
Development and Protective Efficacy of a Novel Nanoparticle Vaccine for Gammacoronavirus Avain Infectious Bronchitis Virus
Abstract
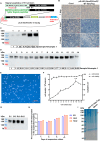
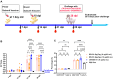
Background: Infectious bronchitis virus (IBV) is a gammacoronavirus that causes a highly contagious disease in chickens and seriously endangers the poultry industry. The GI-19 is a predominant lineage. However, no effective commercially available vaccines against this virus are available. Methods: In this present study, the CHO eukaryotic and the E.coli prokaryotic expression system were used to express S1-SpyTag and AP205-SpyCatcher, respectively. Subsequently, the purified S1-SpyTag and AP205-SpyCatcher were coupled to form the nanoparticles AP205-S1 (nAP205-S1) in PBS buffer at 4 °C for 48 h. S1-SpyTag and nAP205-S1 were formulated into vaccines with white oil adjuvant and employed to immunize 1-day-old SPF chickens for the comparative evaluation of their immune efficacy. Results: The nAP205-S1 vaccine in chickens induced robust IBV-specific humoral and cellular immune responses in vivo. Importantly, the humoral and cellular immune responses elicited by the nAP205-S1 vaccine were more robust than those induced by the IBV S1-SpyTag vaccine at both the same dose and double the dose, with a notably significant difference observed in the cellular immune response. Furthermore, experimental data revealed that chicken flocks vaccinated with nAP205-S1 achieved 100% group protection following a challenge, exhibiting a potent protective immune response and effectively inhibiting viral shedding. Conclusions: These results reveal the potential of developing a novel nanoparticle vaccine with broadly protective immunity against GI-19 IBV.
Keywords: IBV; nanoparticle vaccine; protective efficacy.
Conflict of interest statement
Authors Yanfen Lyu and Ruiai Chen were employed by the company Zhaoqing Dahuanong Biology Medicine Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Xiong T., Xie H., Li L., Liang S., Huang M., Yu C., Zhuang T., Liang X., Liu D., Chen R. Prevalence, Genotype Diversity, and Distinct Pathogenicity of 205 Gammacoronavirus Infectious Bronchitis Virus Isolates in China during 2019–2023. Viruses. 2024;16:930. doi: 10.3390/v16060930. - DOI - PMC - PubMed
Grants and funding
- 2021B0707010009 and 2022B1111040001/Guangdong Provincial Scientific Research Institutions Key Areas R&D Plans
- XJGDL2022002/Science and technology plan projects funded by self-raised funds of Guangdong Provincial Laboratory
- XJ2025T02S01 and XJ2025T02S02/Zhaoqing Branch Center of Guangdong Laboratory for Lingnan Modern Agricultural Science and Technology
LinkOut - more resources
Full Text Sources

