Degradation of Poliovirus Sabin 2 Genome After Electron Beam Irradiation
- PMID: 40872910
- PMCID: PMC12390167
- DOI: 10.3390/vaccines13080824
Degradation of Poliovirus Sabin 2 Genome After Electron Beam Irradiation
Abstract
Objectives: Most antiviral vaccines are created by inactivating the virus using chemical methods. The inactivation and production of viral vaccine preparations after the irradiation of viruses with accelerated electrons has a number of significant advantages. Determining the integrity of the genome of the resulting viral particles is necessary to assess the quality and degree of inactivation after irradiation.
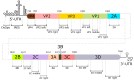
Methods: This work was performed on the Sabin 2 model polio virus. To determine the most sensitive and most radiation-resistant part, the polio virus genome was divided into 20 segments. After irradiation at temperatures of 25 °C, 2-8 °C, -20 °C, or -70 °C, the amplification intensity of these segments was measured in real time.
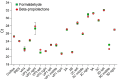
Results: The best correlation between the amplification cycle and the irradiation dose at all temperatures was observed for segment 3D, left. Consequently, this section of the poliovirus genome is the least resistant to the action of accelerated electrons and is the most representative for determining genome integrity. The worst dependence was observed for the VP1 right section, which, therefore, cannot be used to determine genome integrity during inactivation. The electrochemical approach was also employed for a comparative assessment of viral RNA integrity before and after irradiation. An increase in the irradiation dose was accompanied by an increase in signals indicating the electrooxidation of RNA heterocyclic bases. The increase in peak current intensity of viral RNA electrochemical signals confirmed the breaking of viral RNA strands during irradiation. The shorter the RNA fragments, the greater the peak current intensities. In turn, this made the heterocyclic bases more accessible to electrooxidation on the electrode.
Conclusions: These results are necessary for characterizing the integrity of the viral genome for the purpose of creating of antiviral vaccines.
Keywords: accelerated electrons; biosensor; electrochemical analysis; genome degradation; poliomyelitis virus.
Conflict of interest statement
Authors Sergei V. Budnik, Oleg A. Shilov, and Roman S. Churyukin were employed by the company Teocortex LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Piniaeva A., Ignatyev G., Kozlovskaya L., Ivin Y., Kovpak A., Ivanov A., Shishova A., Antonova L., Khapchaev Y., Feldblium I., et al. Immunogenicity and Safety of Inactivated Sabin-Strain Polio Vaccine “PoliovacSin”: Clinical Trials Phase I and II. Vaccines. 2021;9:565. doi: 10.3390/vaccines9060565. - DOI - PMC - PubMed
-
- Polio Eradication Strategy 2022–2026: Executive Summary. World Health Organization; Geneva, Switzerland: 2021.
-
- Bakker W.A.M., Thomassen Y.E., van’t Oever A.G., Westdijk J., van Oijen M.G.C.T., Sundermann L.C., van’t Veld P., Sleeman E., van Nimwegen F.W., Hamidi A., et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine. 2011;29:7188–7196. doi: 10.1016/j.vaccine.2011.05.079. - DOI - PubMed
-
- Sanders B., Koldijk M., Schuitemaker H. Vaccine Analysis: Strategies, Principles, and Control. Springer; Berlin/Heidelberg, Germany: 2014. Inactivated Viral Vaccines; pp. 45–80.
Grants and funding
LinkOut - more resources
Full Text Sources

