Real-World Effectiveness of Omalizumab Treatment in Adult Asthma Patients
- PMID: 40873141
- PMCID: PMC12394757
- DOI: 10.3349/ymj.2024.0320
Real-World Effectiveness of Omalizumab Treatment in Adult Asthma Patients
Abstract
Purpose: Omalizumab improves clinical outcomes for patients with severe asthma (SA), but its long-term effectiveness and potential biomarkers for predicting patient response require further investigation. This study aimed to evaluate the real-world effectiveness of omalizumab in treating SA and to identify potential biomarkers for predicting a favorable treatment response.
Materials and methods: Clinical outcomes were compared between asthma patients receiving omalizumab (omalizumab group) and those on inhaled corticosteroid with long-acting beta-agonist (ICS-LABA) alone (ICS-LABA group). Propensity score matching and Cox proportional hazards model were used to calculate hazard ratios (HRs). Study outcomes included severe asthma exacerbation (SAE), incompletely controlled asthma, intravenous (IV) corticosteroid use, and asthma-related hospitalization. Incompletely controlled asthma was defined by blood eosinophil counts ≥150 cells/µL, fractional exhaled nitric oxide (FeNO) ≥25 ppb, forced expiratory volume in one second (FEV1%) <80%, or SAE occurrence.
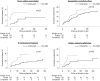
Results: The omalizumab group had significantly lower risks of SAE (HR 0.17, p=0.03), incompletely controlled asthma (HR 0.56, p=0.04), IV corticosteroid treatment (HR 0.38, p=0.02), and asthma-related hospitalization (HR 0.27, p=0.05). Blood eosinophil count stayed lower in the omalizumab group. FEV1% was higher with the omalizumab group, while blood neutrophil count, FeNO, and serum total IgE showed no differences. Furthermore, subgroup analysis showed patients with treatment-favorable response (>50% reduction in systemic corticosteroid dose) exhibited decreased blood neutrophil counts but increased FEV1% and serum total IgE levels compared with the treatment-unfavorable group.
Conclusion: Omalizumab treatment effectively reduces SAE and improves lung function and asthma control. Blood neutrophil counts and serum total IgE may be potential biomarkers for predicting favorable responses to omalizumab treatment.
Keywords: Asthma; IgE; neutrophils; omalizumab.
© Copyright: Yonsei University College of Medicine 2025.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures




Similar articles
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Omalizumab for asthma in adults and children.Cochrane Database Syst Rev. 2014 Jan 13;2014(1):CD003559. doi: 10.1002/14651858.CD003559.pub4. Cochrane Database Syst Rev. 2014. PMID: 24414989 Free PMC article.
-
Exhaled nitric oxide levels to guide treatment for children with asthma.Cochrane Database Syst Rev. 2016 Nov 9;11(11):CD011439. doi: 10.1002/14651858.CD011439.pub2. Cochrane Database Syst Rev. 2016. PMID: 27825189 Free PMC article.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.Health Technol Assess. 2008 May;12(20):1-174, iii-iv. doi: 10.3310/hta12200. Health Technol Assess. 2008. PMID: 18485272
-
Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children.Cochrane Database Syst Rev. 2015 Nov 24;2015(11):CD007949. doi: 10.1002/14651858.CD007949.pub2. Cochrane Database Syst Rev. 2015. PMID: 26594816 Free PMC article.
References
-
- Chervinsky P, Casale T, Townley R, Tripathy I, Hedgecock S, Fowler-Taylor A, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91:160–167. - PubMed
-
- Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2019;7:156–164.e1. - PubMed

