KCTD10 inhibits lung cancer metastasis and angiogenesis via ubiquitin-mediated β-catenin degradation
- PMID: 40873559
- PMCID: PMC12378768
- DOI: 10.3389/fimmu.2025.1630311
KCTD10 inhibits lung cancer metastasis and angiogenesis via ubiquitin-mediated β-catenin degradation
Abstract
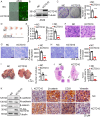
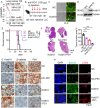
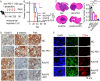
Lung cancer remains a critical global health concern, characterized by the highest incidence and mortality rates among all cancers. Due to its heterogeneity and complexity, the molecular mechanism underlying lung cancer occurrence and progression needs to be further investigated. KCTD10 has been implicated in malignant phenotypes of several tumors, but the role of KCTD10 in lung cancer remains largely unexplored. In this study, we found that KCTD10 expression is significantly reduced in lung cancer tissues, and overexpression of KCTD10 could inhibit lung cancer progression both in vitro and in vivo. Immunoprecipitation-mass spectrometry (IP-MS), co-immunoprecipitation (Co-IP), and ubiquitination assays revealed that the BTB domain of KCTD10 interacts with Armadillo repeat domains 1-9 of β-catenin and facilitates ubiquitin-dependent degradation of β-catenin via the K48-linked ubiquitin chains, followed by the downregulation of the β-catenin downstream target gene PD-L1. Notably, the combined treatment of KCTD10 overexpression with anti-PD-1 antibodies exhibited a synergistic effect in suppressing lung cancer progression and brain metastatic colonization in mice. In addition, vascular endothelial cell-specific knockout of Kctd10 (Kctd10flox/floxCDH5CreERT2/+) promoted lung cancer metastasis and tumor angiogenesis through β-catenin signaling. Finally, we identified METTL14- mediated N6-methyladenosine (m6A) modification within the coding sequence (CDS) region of KCTD10, which enhanced KCTD10 mRNA stability in a YTHDF2-dependent manner. These findings highlight KCTD10 as a critical regulator of lung cancer progression and the tumor microenvironment, suggesting its potential as a promising therapeutic target for lung cancer.
Keywords: KCTD10; M6A; PD-1; lung cancer metastasis; specific Kctd10 knockout; β-catenin.
Copyright © 2025 Yin, Long, Zhou, Ouyang, Wang, He, Su, Li, Ding and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

