Smart CAR-T Nanosymbionts: archetypes and proto-models
- PMID: 40873579
- PMCID: PMC12379055
- DOI: 10.3389/fimmu.2025.1635159
Smart CAR-T Nanosymbionts: archetypes and proto-models
Abstract
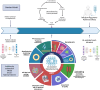
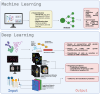
Personalized medicine has redefined cancer treatment by aligning therapies with each patient's unique biological profile. A key example is chimeric antigen receptor T-cell (CAR-T) therapy, in which a patient's own T cells are genetically modified to recognize and destroy cancer cells. This approach has delivered remarkable results in hematologic malignancies and is beginning to show promise in solid tumors and autoimmune diseases. However, its broader adoption is limited by major challenges, including complex manufacturing, high costs, limited efficacy in solid tumors, and potentially severe toxicities. Nanotechnology offers exciting possibilities to overcome many of these barriers. Engineered nanoparticles can improve gene delivery, target tumors more precisely, enhance immune cell function, and enable in vivo CAR-T production, reducing the need for labor-intensive ex vivo processes. However, despite this promise, translation into clinical settings remains difficult due to regulatory hurdles, scalability issues, and inconsistent reproducibility in human models. At the same time, artificial intelligence (AI), with its powerful algorithms for data analysis and predictive modeling, is transforming how we design, evaluate, and monitor advanced therapies, including the optimization of manufacturing processes. In the context of CAR-T, AI holds strong potential for better patient stratification, improved prediction of treatment response and toxicity, and faster, more precise design of CAR constructs and delivery systems. Leveraging these three technological pillars, this review introduces the concept of Smart CART Nanosymbionts, an integrated framework in which AI guides the design and deployment of nanotechnology-enhanced CAR-T therapies. We explore how this convergence enables optimization of lipid nanoparticle formulations for mRNA transfection, specific targeting and modification of the tumor microenvironment, real-time monitoring of CAR-T cell behavior and toxicity, and improved in vivo CAR-T generation and overcoming barriers in solid tumors. Finally, it's important we also address the ethical and regulatory considerations surrounding this emerging interface of living therapies and computational driven systems. The Smart CART Nanosymbionts framework (Figure 1:) represents a transformative step forward, promising to advance personalized cancer treatment toward greater precision, accessibility, and overall effectiveness.
Keywords: CAR-T therapy; artificial intelligence; deep learning; immunotherapy; machine learning; manufacturing; nanotechnology; personalized medicine.
Copyright © 2025 Baena, Victoria, Toro-Pedroza, Aragón, Ortiz-Guzman, Garcia-Robledo, Torres, Rios-Serna, Albornoz, Rosales, Cañas, Adolfo Cruz-Suarez, Osorio, Fleitas, Laponogov, Loukanov and Veselkov.
Conflict of interest statement
Author JO-G is the founder and CEO of Prodigy Cells Labs, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Personalized Medicine. Available online at: https://www.genome.gov/genetics-glossary/Personalized-Medicine (Accessed March 21, 2025).
-
- Commissioner O of the FDA . FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma(2020). Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-c... (Accessed March 28, 2025).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

