Enhanced Blood-Brain Barrier Penetrability of BACE1 SiRNA-Loaded Prussian Blue Nanocomplexes for Alzheimer's Disease Synergy Therapy
- PMID: 40873646
- PMCID: PMC12380061
- DOI: 10.1002/EXP.20230178
Enhanced Blood-Brain Barrier Penetrability of BACE1 SiRNA-Loaded Prussian Blue Nanocomplexes for Alzheimer's Disease Synergy Therapy
Abstract
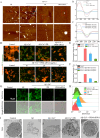
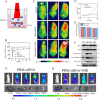
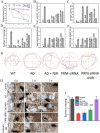
Amyloid-β (Aβ) deposition was an important pathomechanisms of Alzheimer's disease (AD). Aβ generation was highly regulated by beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), which is a prime drug target for AD therapy. The silence of BACE1 function to slow down Aβ production was accepted as an effective strategy for combating AD. Herein, BACE1 interfering RNA, metallothionein (MT) and ruthenium complexes ([Ru(bpy)2dppz]2+) were all loaded in Prussian blue nanoparticles (PRM-siRNA). PRM-siRNA under near-infrared light irradiation showed good photothermal effect and triggered instantaneous opening of blood-brain barrier (BBB) for enhanced drug delivery. BACE1 siRNA slowed down Aβ production and Cu2+ chelation by metallothionein (MT) synergistically inhibited Aβ aggregation. Ruthenium (Ru) could real-timely track Aβ degradation and aggregation. The results indicated that PRM-siRNA significantly blocked Aβ aggregation and attenuated Aβ-induced neurotoxicity and apoptosis in vitro by inhibiting ROS-mediated oxidative damage and mitochondrial dysfunction through regulating the Bcl-2 family. PRM-siRNA in vivo effectively improved APP/PS1 mice learning and memory by alleviating neural loss, neurofibrillary tangles and activation of astrocytes and microglial cells in APP/PS1 mice by inhibiting BACE1, oxidative damage and tau phosphorylation. Taken together, our findings validated that BACE1 siRNA-loaded Prussian blue nanocomplexes showed enhanced BBB penetrability and AD synergy therapy.
Keywords: Alzheimer's disease; BACE1; Prussian blue nanoparticles; amyloid‐β; blood‐brain barrier.
© 2025 The Author(s). Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Ali F. E., Separovic F., Barrow C. J., Yao S., and Barnham K. J., “Copper and Zinc Mediated Oligomerisation of Aβ Peptides,” International Journal of Peptide Research and Therapeutics 12 (2006): 153–164.
-
- Behbehani G. R., “A Novel Method for Thermodynamic Study on Binding of Copper Ion With Alzheimer's Amyliod β Peptide,” Chinese Science Bulletin 54 (2009): 1037–1042.
-
- Epa V. C., Streltsov V. A., and Varghese J. N., “Modelling Copper Binding to the Amyloid‐β Peptide in Alzheimer,” Australian Journal of Chemistry 63 (2010): 345–349.
-
- Isaev N. K., Stelmashook E. V., and Genrikhs E. E., “Role of Zinc and Copper Ions in the Pathogenetic Mechanisms of Traumatic Brain Injury and Alzheimer's Disease,” Reviews Neuroscience 31 (2020): 233–243. - PubMed
LinkOut - more resources
Full Text Sources
