Impact of multi-strain probiotics supplementation on growth, immune responses and physiological traits in backyard poultry of Andaman and Nicobar Islands, India
- PMID: 40873715
- PMCID: PMC12379071
- DOI: 10.3389/fmicb.2025.1625167
Impact of multi-strain probiotics supplementation on growth, immune responses and physiological traits in backyard poultry of Andaman and Nicobar Islands, India
Abstract
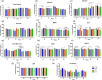
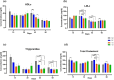
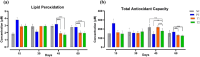
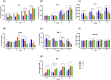
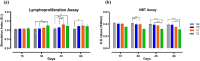
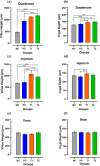
Antibiotic growth promoters (AGPs) are widely used as feed additives to enhance the immunity, and productivity in the poultry industries. But over usage of AGPs has led to multi drug-resistance among pathogens. Nonspecific immunomodulators like probiotics have emerged as competent replacements of AGPs. Probiotics plays a key role in gut microbial health by its mechanism of action and modulation of host immune system. No prior research has been conducted in the Andaman and Nicobar Islands, India to elucidate the direct influence of probiotics on health and immunity of backyard poultry. To explore an efficient alternative to AGP, a commercial multi strain probiotics (Bifilac R ) was evaluated in Vanaraja, a popular backyard poultry breed reared in the islands. 120 newly hatched Vanaraja chicks were chosen, 30 chicks were randomly allocated into 4 different groups for 60 days. For the negative control (NC), chicks were fed only basal diet. For the positive control (PC), chicks were fed with basal diet + AGP (Tetracycline). Test group (T1) was fed basal diet + 0.1% of Bifilac R . The test group (T2) was fed basal diet + 0.3% of Bifilac R . The results showed that the mean body weight of chicks supplemented with 0.1% (T1) and 0.3% (T2) of multi-strain probiotics was significantly higher (p ≤ 0.05) compared to the control groups. A significant increase (p ≤ 0.05) in FCR was also observed among T1 and T2 at different time intervals. Both T1 and T2 expressed significant changes (p ≤ 0.05) in biochemical parameters such as albumin, globulin, BUN, total bilirubin, SGOT and SGPT at different time intervals than the control groups. A significant decrease (p ≤ 0.05) was noticed in T1 and T2 groups in the levels of triglycerides, HDLc, LDLc, total cholesterol, superoxide production, lipid peroxidation at different time intervals. A significant increase (p ≤ 0.05) was observed in the levels of HSP70, IL4, IL2, and lymphocyte proliferation in T1 and T2 compared to the control groups. After histomorphological analysis, an increase (p ≤ 0.001) in villus height (μm) and crypt depth (μm) in duodenum and jejunum were noticed in T1 and T2. In short, multi-strain probiotics supplementation showed its potential as an overall growth promoter in terms of improved growth performance, favorable physiological functions, enhanced immunomodulatory effects and better intestinal morphology in a widely reared backyard poultry breed of Andaman and Nicobar Islands, India, hence can be nominated as a potential alternative to commercial antibiotics at ground level.
Keywords: Andaman and Nicobar Islands; backyard poultry; growth promoter; immunomodulation; multi-strain probiotics; serum biochemistry.
Copyright © 2025 Halder, Joardar, Sunder, De, Bhattacharya, Abd El Wahed, Kobialka, Madanan and Mondal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abdel-Moneim A., Selim D., Basuony H., Sabic E., Saleh A., Ebeid T. (2020). Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 52 671–680. 10.1007/s11250-019-02055-1 - DOI - PubMed
-
- Abudabos A., Alhouri H., Alhidary I., Nassan M., Swelum A. (2019). Ameliorative effect of Bacillus subtilis, Saccharomyces boulardii, oregano, and calcium montmorillonite on growth, intestinal histology, and blood metabolites on Salmonella-infected broiler chicken. Env. Sci. Pollut. Res. Int. 26 16274–16278. 10.1007/s11356-019-05105-1 - DOI - PubMed
-
- Adil S., Magray S. (2012). Impact and manipulation of gut microflora in poultry: A review. J. Anim. Vet. Adv. 11 873–877. 10.3923/javaa.2012.873.877 - DOI
-
- Ahmed E., Abdelrahman M., Gahreeb K. (2019). Effect of probiotic on growth performance, carcass traits, and clinical health parameters of broilers reared under heat stress in upper Egypt. SVU-Int. J. Vet. Sci. 2 27–44. 10.21608/svu.2019.11221.1012 - DOI
-
- Alimohamadi K., Taherpour K., Ghasemi H., Fatahnia F. (2014). Comparative effects of using black seed. (Nigella sativa), cumin seed. (Cuminum cyminum), probiotic or prebiotic on growth performance, blood haematology and serum biochemistry of broiler chicks. J. Anim. Physiol. Anim. Nutr. 98 538–546. 10.1111/jpn.12115 - DOI - PubMed
LinkOut - more resources
Full Text Sources

