Antimicrobial activity of Pediococcus pentosaceus strains against diarrheal pathogens isolated from pigs and effect on paracellular permeability of HT-29 cells
- PMID: 40873998
- PMCID: PMC12380015
- DOI: 10.5187/jast.2024.e47
Antimicrobial activity of Pediococcus pentosaceus strains against diarrheal pathogens isolated from pigs and effect on paracellular permeability of HT-29 cells
Abstract
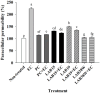
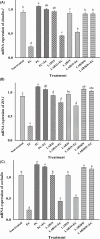
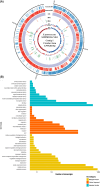
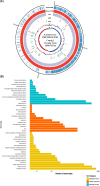
This study aimed to investigate lactic acid bacteria with antimicrobial activities against infectious diarrheal pathogens in pigs and their genetic characteristics. Acid-resistant lactic acid bacteria were examined for bile resistance, pancreatic enzyme resistance, gelatinase and urease activities, and antibiotic resistance. Subsequently, selected isolates were examined for antimicrobial activities against Campylobacter coli, Clostridium perfringens, Escherichia coli, and Salmonella Typhimurium, and their effects on paracellular permeability and the expression of tight junction protein-encoding genes in HT-29 cells were assessed. Whole genome sequencing was performed to identify the genes related to safety and antibacterial activity. Of the 51 isolates examined, 12 were resistant to bile and pancreatin and did not produce gelatinase and urease. Of these 12, isolates 19, 20, 30, 36, and 67 showed tetracycline resistance and isolates 15, 19, and 38W showed antimicrobial activity against infectious diarrheal bacteria. Treatment with isolate 38W significantly reduced the paracellular permeability induced by E. coli in HT-29 cells and alleviated the expression of tight junction protein-encoding genes (claudin-1, occludin, and ZO-1) induced by E. coli inoculation. Isolates 15, 19, and 38W were named as Pediococcus pentosaceus SMFM2016-NK1, SMFM2016-YK1, and SMFM2016-WK1, respectively. Bacteriocin-related genes were YheH, ytrF, BceA, BceB, and MccF in SMFM2016-NK1; YheH, ytrF, BceA, BceB, entK, lcnA, MccF, and skgD in SMFM2016-YK1; and YheH, ytrF, BceA, BceB, and MccF in SMFM2016-WK1. SMFM2016-YK1 harbored the tetM gene. These results indicate that P. pentosaceus SMFM2016-WK1 might control diarrheal pathogens isolated from pigs. However, a further study is necessary because the results were obtained only from in vitro experiment.
Keywords: Antimicrobial agent; Feed additive; Gut health; Lactic acid bacteria; Probiotics.
© Copyright 2025 Korean Society of Animal Science and Technology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Antimicrobial activity of lactic acid bacteria isolated from the gut of African catfish (Clarias gariepinus) against multi-drug-resistant resident pathogens.BMC Microbiol. 2025 Jul 4;25(1):410. doi: 10.1186/s12866-025-04139-5. BMC Microbiol. 2025. PMID: 40615981
-
In Vitro Characterization and Safety Assessment of Streptococcus salivarius, Levilactobacillus brevis and Pediococcus pentosaceus Isolated from the Small Intestine of Broiler Breeders.Microorganisms. 2025 May 27;13(6):1231. doi: 10.3390/microorganisms13061231. Microorganisms. 2025. PMID: 40572119 Free PMC article.
-
A systematic review on natural products with antimicrobial potential against WHO's priority pathogens.Eur J Med Res. 2025 Jul 1;30(1):525. doi: 10.1186/s40001-025-02717-x. Eur J Med Res. 2025. PMID: 40597250 Free PMC article.
-
Evaluation of probiotic potential, safety assessment and whole genome sequencing of Lactiplantibacillus plantarum strain MOVIN isolated from toddy sample.Front Microbiol. 2025 Jul 10;16:1625659. doi: 10.3389/fmicb.2025.1625659. eCollection 2025. Front Microbiol. 2025. PMID: 40708923 Free PMC article.
-
Probiotics for preventing urinary tract infection in people with neuropathic bladder.Cochrane Database Syst Rev. 2017 Sep 8;9(9):CD010723. doi: 10.1002/14651858.CD010723.pub2. Cochrane Database Syst Rev. 2017. PMID: 28884476 Free PMC article.
References
-
- Thomson JR, Friendship RM. Digestive system. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Diseases of swine. 11th ed. Hoboken, NJ: Wiley & Sons; 2019. pp. 234–63.
-
- Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave Commun. 2018;4:96. doi: 10.1057/s41599-018-0152-2. - DOI
LinkOut - more resources
Full Text Sources

