Retinal Vessel Changes in Geographic Atrophy in AMD: Insights From Imaging and Histology
- PMID: 40874698
- PMCID: PMC12400968
- DOI: 10.1167/iovs.66.11.69
Retinal Vessel Changes in Geographic Atrophy in AMD: Insights From Imaging and Histology
Abstract
Purpose: The purpose of this study was to investigate retinal vascular changes in geographic atrophy (GA) secondary to age-related macular degeneration (AMD) using swept-source optical coherence tomography angiography (SS-OCTA), and to correlate imaging findings with histology.
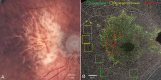
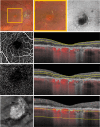
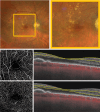
Methods: Sixty subjects were enrolled: 20 with GA, 20 with intermediate AMD, and 20 healthy controls. SS-OCTA imaging was used to quantify retinal perfusion density (PD) and vessel length density (VLD) in the superficial capillary plexus (SCP), deep capillary plexus (DCP), and full retina. A topographical analysis distinguished regions with and without retinal pigment epithelium (RPE) atrophy in GA eyes. Additionally, flat-mount immunohistochemistry was performed on a donor eye with GA to assess retinal vasculature. Main outcome measures included PD and VLD across SCP, DCP, and full retina, in regions with and without RPE atrophy.
Results: Retinal PD and VLD were significantly reduced in GA eyes compared with intermediate AMD eyes, particularly in the DCP. Topographical analysis revealed more pronounced vascular impairment in areas with RPE atrophy, whereas regions without RPE atrophy in GA eyes exhibited perfusion comparable to intermediate AMD and healthy controls. Histological analysis confirmed a substantial reduction in vascular density within atrophic regions.
Conclusions: Retinal vascular changes in GA predominantly occur within regions of RPE atrophy. The preservation of perfusion in regions without RPE atrophy suggests that vascular impairment is localized. These findings underscore the importance of regional analysis and histopathologic correlation in understanding vascular remodeling in GA. Future longitudinal OCTA studies are warranted to clarify the temporal progression of these vascular alterations in relation to RPE atrophy.
Conflict of interest statement
Disclosure:
Figures


References
-
- Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2020; 127: S122–S132. - PubMed
-
- Dolz-Marco R, Balaratnasingam C, Messinger JD, et al.. The border of macular atrophy in age-related macular degeneration: a clinicopathologic correlation. Am J Ophthalmol. 2018; 193: 166–177. - PubMed
-
- Fleckenstein M, Keenan TDL, Guymer RH, et al.. Age-related macular degeneration. Nat Rev Dis Primers. 2021; 7: 31. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

